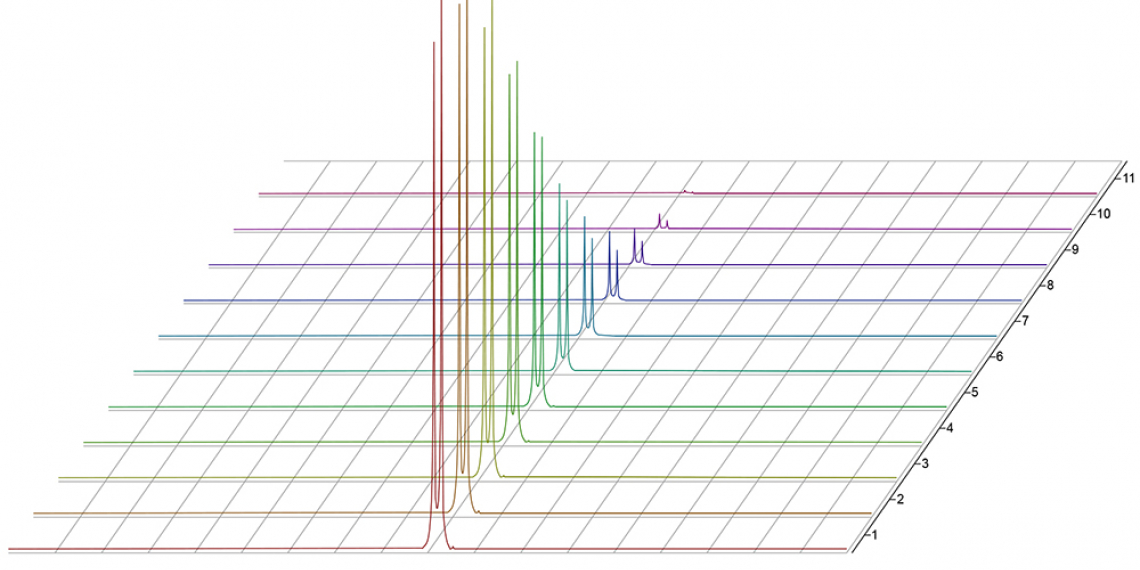
Benchtop NMR enhances electrolyte design by enabling rapid and routine measurement of key performance parameters including electrolyte composition, diffusion coefficients of each component, conductivity, transference number and viscosity. Hence, it provides critical data for the design of new electrolyte formulations and optimisation of their performance. This application report outlines practical approaches for NMR electrolyte analysis and how the measured parameters can affect performance using examples from lithium battery R&D.
Electrolytes, which consist of anions and cations in a solvent system, are a key component in battery performance. Rechargeable lithium-ion (Li-ion) batteries offer high energy density and have become extremely popular, providing energy storage for electronics, medical devices and electric vehicles. Current battery technologies use small-molecule liquid organic solvents, such as ethylene carbonate (EC), dimethyl carbonate (DMC), coupled with small Li+ and hexafluorophosphate ([PF6]−) ionic species. However, new electrode formulations such as Li-metal, lithiated silicon or lithium sulfur, require new electrolyte chemistries. Optimising the electrolyte performance delivers improvements in power output, longevity and safety, making development of new systems a high priority.

