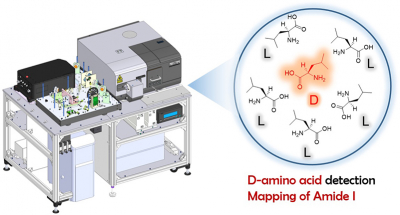
Currently, the roles of free D-amino acids and D-amino acid residues in proteins are garnering extensive attention in biological fields such as molecular biology, physiology, microbiology and pathophysiology. Since it is crucial to analyse these materials rapidly and accurately, many methods have been used. However, samples for measurement are currently limited to solutions containing target molecules in the pure form. Hence, there is need for an analytical method for the in situ measurement of biological samples placed on a solid support.
Scientists at have Ehime University have constructed a multidimensional vibrational circular dichroism spectroscopy (MutilD-VCD) for dipeptides and amino acids with D-amino acid residues in amides I and II regions. A quantum cascade laser (QCL) was installed as an infrared source to achieve high intensity and narrow focusing. A MultiD-VCD system with three coordinates (wavenumber, VCD intensity and position) was developed. The instrument was switchable to a concurrent FT-VCD (Fourier transform vibrational circular dichroism) system. A scanning device was installed to move an attached motorised stage in two dimensions.
Results indicated that the instrument could obtain the VCD spectrum of dipeptides, including D-amino acid, in an aqueous solution under interference caused by a water medium. Moreover, it achieved the two-dimensional patterning of the distribution and discrimination of chiral components (L-Ala and D-Ser) within a KBr pellet. Our final goal is to identify the location of D-amino acid residue in the aggregates of proteins, such as amyloids, and to clarify how the chiral inversion of a single amino acid residue in a protein affects its biological functions. An improved spatial resolution may benefit the basic fields of analytical and medical sciences as well as the practical field of chiral component analysis in drugs in the future.