Peter J. Jenksa and John P. Hammondb
aThe Jenks Partnership, Newhaven House, Junction Road, Alderbury, Salisbury, Wiltshire SP5 3AZ, UK
bStarna Scientific Ltd, 52–54 Fowler Road, Hainault Business Park, Hainault, Essex, IG6 3UT, UK
In part one, published in Spectroscopy Europe 24(3), p. 26 I looked at the role of CRMs and PT in an ISO 17025 accredited laboratory. In this part John Hammond and I will follow up on what a Laboratory Manager can do to provide a suitable alternative to a CRM when one is unavailable. But, first, a couple of interesting and in one case important developments.
Some readers will remember the newsletter RM Report. In May/June 2003 I interviewed Dr Martin Milton, then Group Leader for Gas Metrology at the UK National Physical Laboratory. Since then he has worked diligently to advance not just gas metrology in Europe but to advance the status of European metrology within the world metrological community. It is with great pride that I learn that Martin has been appointed Deputy Director of the Bureau International des Poids et Mesures (BIPM), or in English the International Bureau of Weights and Measures.
The BIPM is the global authority on all things that relate to weights and measures and is the “home” of the SI Convention. Martin will join the BIPM on 1 October 2012 as Deputy Director/Director Designate to take over the directorship from the present Director, Dr Michael Kühne, on 1 January 2013. In addition Dr Brian Bowsher (Managing Director, NPL, UK) has been appointed to the Comité International des Poids et Mesures (CIPM), or International Committee of weights and measures.
These appointments show just how highly European Metrology and European Science are regarded on the world stage, something to be very proud of.
Another notable event, of sorts, concerns this column. Back in 2001 I first talked with the Publisher about a regular column looking at reference materials and quality matters: we agreed to give it a try and 11 years later this is the 50th column I’ve written for Spectroscopy Europe! Over the years I’ve been aided and assisted by a number of guest contributors and more recently by Chris Burgess and John Hammond and between us we have tried to inform and stimulate our readers.—PJJ
Part 2, in-house certified reference materials (IHCRM)
If it is impossible to find a CRM that is appropriate for the intended application, the simplistic answer is to produce an IHCRM. This is a much bigger task than producing an in-house reference material (IHRM) or standard. Outside the pharmaceutical industry, where production of secondary reference (working) standards is permitted by direct comparison with the primary and without extensive characterisation, it is a complex task.
Also, in the discussions shown below, the assumption is that the value assignment(s) produced in the certification for the reference material will be for quantitative assigned numerical value(s), as opposed to qualitative, “nominal” value(s).
ISO REMCO has recognised this difficulty and is in the process of developing a new guide: Guide 80: Minimum Requirements for the Production of In-House Reference Materials. But it is not yet approved or available and even when “official” they will only assist the IHRM producer and subsequent discussions with Accreditation Bodies. For now, Guide 34 is the key.
The main difficulty is that to meet the requirement of an external accreditor the in-house CRM has to be effectively “certified” according to the requirements of ISO Guide 34, so to a standard that is almost the same as the certification developed and supported by specialist producers.
What does this mean? This article reviews the steps that have to be followed to set up a procedure to produce in-house CRMs.
The first and most important step is to get hold of three of the ISO REMCO Guides, Guide 34 is the key but Guide 35 is important, as is Guide 31. The ISO REMCO Guides are not free and can be ordered from http://www.iso.org/iso/home/store/catalogue_tc/catalogue_tc_browse.htm?commid=55002
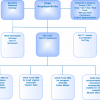
The ISO Guides work together to provide a framework around which RMs and CRMs may be produced, as Figure 1 shows.

Note: ISO Guide 79 is a specific guidance document (in preparation) for the production of reference materials where the value assignment is for a qualitative, or “nominal” property, and therefore only applies in this specific case.
Once your background reading is completed you must prepare a written quality manual that sets out what you plan to do.
Organisations already accredited to ISO 17025 will be familiar with this requirement, and in fact one of the major areas of revision with the latest version of ISO Guide 34 was to align it with the current version of ISO 17025. Therefore, these organisations recognise many of the requirements, but there are obviously specific requirements for reference material production, and these are detailed below. If you have a Quality Manager available to provide guidance and support it will be a big help.
Now you are ready to start!
First, find one or more reliable, but different, sources of the material you plan to use as candidate materials. But how can you be sure they are really independent? Do not assume that if you buy the same material from two different suppliers it is different, for many substances, especially complex organic molecules, there may only be one true source.
Check that the bulk material is homogeneous. This is relatively easy if it is in solution, but much harder if it is a powder. The usual requirement is make sure that the material has a uniform particle size and then mix, using a “V” blender, for an extended period. To prove the mixing has been effective you must pick two or three “marker” analytes and sample through the mixed material, then analyse the marker analytes and carry out a statistical evaluation.
Once you are satisfied it is homogeneous you need to pack all the bulk into final use packs, each one should contain no more than you will use up in two weeks, less if the sample is hygroscopic or in any way unstable. You want sufficient units to last two or three years, after all you do not want to do all this often.
Now check that the between pack homogeneity is acceptable, that is all the units are the same. To do this take two or three random units from the first, middle and last third of the packing run and analyse for your marker analytes. If the check analytes are OK then do some basic analytical characterisation, for example, if your material contains an organic profile run and IR, and compare.
Now it is time to start the analytical phase. The ideal is 11 sets of data, each from a different sample. If possible, more than one analyst should be used and different instruments. For perfection, organise a proper inter-laboratory study, but this is not often possible. For organic molecules if you can assay using GC-MS/MS try to identify impurities and quantify them and if you have access to quantitative NMR (qNMR) then you can really start to develop good data.
Once you have amassed a suitable quantity of data, use basic robust statistics to calculate an analytical mean, standard deviation and if possible calculate a certified value. ISO Guide 35 is the reference for everything to do with statistics, it is not an easy read and you will need a good understanding of statistics.
As you develop the analytical data you need to investigate the stability of the material, published data may help, and set an expiry/re-test date. But you have to prove your assigned expiry date and re-test plans. To do this it can be helpful to stress test the samples by using elevated temperature, light levels and humidity.
Once your statistics are completed, and assuming the results and associated uncertainty are fit for purpose you can prepare Certificate of Analysis (CoA). Preparing a CoA is not easy: to comply with the requirements of ISO Guide 31, which is referenced in ISO Guide 34. A CoA for a CRM should contain all of the following:
- Name of the material,
- Producer’s name, producer’s product code and lot number,
- General description of the material,
- Intended use,
- Instructions for proper use, including minimum sample size to be taken,
- Storage instructions and shelf life for the UNOPENED unit,
- Certified property values, with uncertainty,
- Methods used to obtain certified value, especially if values are dependent on the analytical method,
- Certificate validity, if different to shelf life,
- Name of certifying officer (signature optional!),
- Accreditation that applies, if any.
When this is done you have a certified reference material with a value that is probably reliable and may convince your external auditor it is fit for use as a CRM.
As the above overview makes very clear there is a lot of work associated with producing an IHCRM and it is why buying in a commercially produced CRM is often cheaper and easier.
Producing an IHCRM is demanding and the external technical assessor appointed by the accreditation service may need to be convinced that your procedures are acceptable, before you start. So before starting on this journey it may be best to talk to all the possible accredited suppliers. It may be that one of them is already working on a CRM that meets your needs. If not many have the ability to offer custom calibration CRMs as long as the analytes and matrix are covered by the producer’s scope of accreditation.
Part 3, in-house proficiency testing samples
It all gets rather more challenging when PT is not available. As PT depends on comparing the performance of one laboratory against another it is much harder to go down the “in-house” route.
Essentially you have two options.
Talk to a PT provider: they may already be thinking about offering a service; they may be able to adapt an existing PT product as a “custom”.
Talk to other labs doing the same analysis and see if you can set up a cooperative inter-laboratory study; it helps if you can involve academics, they have graduate students…
Your candidate materials have to be produced just as if they were to be used as IHCRMs. Indeed, you may be able to produce an IHCRM from the PT analytical data!
When you have exhausted all possibilities and can document that you have explored the options then tell your accreditation auditor that you have checked and there is nothing available. If there is no PT possible you do not have to do PT. But remember the accreditation body will expect you to keep checking every three to six months!
Finally, if you are determined to produce and in-house CRM or PT there are a few reference sources that may help:
“The Book”: M. Stoeppler, W.R. Wolf and P.J. Jenks, Reference Materials for Chemical Analysis. Wiley-VCH, Weinheim, Germany (2001).
“The Journal”: Accreditation and Quality Assurance, Springer