Sodium metal batteries (SMBs) have attracted extensive attention because of their high theoretical capacity (1166 mAh g–1), low redox potential (−2.71 V vs SHE), high natural material abundance and low cost. However, the growth of dendrites results in poor battery performance and severe safety problems, inhibiting the commercial application of SMBs. In order to stabilise sodium metal anodes, various methods have been developed to optimise the solid electrolyte interphase (SEI) layer and adjust the electroplating/stripping behaviour of sodium. Among them, developing anode host materials and adding electrolyte additives to build a protective layer are promising and convenient ways. In order to achieve the rational design of advanced anode hosts and electrolyte additives, the understanding of the interaction process between sodium metal and those organic materials is of great significance.
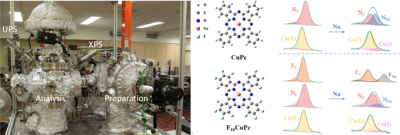
Researchers led by Prof. Wei Chen at National University of Singapore, Singapore, are interested in the interface protection of sodium metal anodes, which is indispensable for the development of sodium metal batteries. They creatively linked in situ interface research methods with the protection of sodium metal anodes. Since the battery system is complicated with various electrolyte compositions and side reactions, in order to simplify the research system and give direct evidence about the interaction process between sodium metal anodes and the electrolyte additives (or hosts), they used organic molecules as model systems. Through their customer-designed in situ UHV X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS) systems, they unravelled the Na interaction process at Na/CuPc and Na/F16CuPc interfaces, especially the effect of fluorination on sodiophilic sites, which provide insights into the radical design of fluorine-containing electrolyte additives and hosts for the protection of sodium metal anodes.