J.P. Hammond
Starna Scientific Limited, 52/54 Fowler Road, Hainault, IG6 3UT, UK. E-mail: [email protected]
Introduction
The 33rd meeting of the Reference Material Committee of ISO, ISO/REMCO was held in Hangzhou (China) from 3 to 7 May 2010, and was hosted by the Standardisation Administration of China and the China Association of Standardisation. ISO/REMCO now has a membership of 70 members of the International Organisation for Standardisation (ISO) and liaison with 18 international organisations and seven ISO-internal committees. The new ISO TC liaison introduced at this meeting is with ISO/TC 158 “Analysis of gases”, with Dr Adriaan van der Veen acting as the REMCO liaison officer.
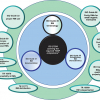
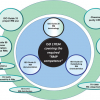
One of the main tasks of ISO/REMCO is preparing guides for the preparation, characterisation, certification and use of reference materials (RMs) and the competence assessment of reference material (RM) producers. These current and proposed guides and their relationships are shown in Figure 1.

ISO/REMCO has updated its work programme during the meeting in Hangzhou. Main activities for 2010 and beyond include:
- a complete revision of the vocabulary related to reference materials (ISO Guide 30, 1992/Amd 1:2008),1 supported by extensive consultation with other liaison organisations;
- a complete revision of the guide on accompanying documentation (ISO Guide 31, 2000),2 expanded to cover all documentation for reference materials;
- a complete revision of the guide on the use of reference materials (ISO Guide 33, 2000);3
- a systematic review of the guide for the characterisation and certification of RMs (ISO Guide 35, 2006);4
- the development of a new guide for the in-house production of in-house used reference materials for quality control (ISO Guide 80);
- the development of a new guide for the production of RMs for “qualitative analysis”—testing of nominal properties (ISO Guide 79);
- further studies into the matter of “metrological traceability”, in particular how to express the concept on CRM certificates and related documentation.
Introductory information to ISO/REMCO
A revised introduction to ISO/REMCO has been produced. This booklet is available on the ISO/REMCO website at http://www.iso.org/iso/remco_2009.pdf and explains the mission and structure of ISO/REMCO, available documents and the system of guides. Moreover, it provides information how to communicate with the committee.
Committee structure
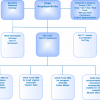
ISO/REMCO 2010 is structured as shown in Figure 2. The organisation consists of Working Groups (WG) under two Steering Groups (SG1 and SG2). The Chairman’s Advisory Group (CAG) provides input to the Chair from the group convenors.

Last year, ISO Guide 34,5 the guide describing the requirements for the quality management system and the technical competences of reference material producers, was fully revised. This 3rd revision aligned the document with ISO/IEC 17025:2005,6,7 and as such there is now the requirement to provide support with respect to education and training in these key areas.
ISO Guide 34 has become the worldwide “standard” for RMP accreditation and was included into the Mutual Recognition Arrangement (MRA)8 of National Metrology Institutes (NMIs) and other signatories of this MRA. The arrangement refers directly to ISO Guides 34 and 355,4 with respect to the requirements for certified reference materials (CRM).
Vocabulary
ISO/REMCO’s vocabulary is in need of revision, and has to clearly define the nuances specific to the use of terminology when applied to RMs.
The term “commutability” was considered, and thought to be important, but there was such a variety of ideas about how the term should be defined that it was deemed prudent to undertake a separate study of the concept. It has been proposed to hold a workshop on the topic in conjunction with the next ISO/REMCO meeting in Delft, July 2011.
Uses of RMs
ISO Guide 333 is also under revision. The current document deals with uses of CRMs, excluding calibration, which is covered by ISO Guide 32.9 As shown in the guide structure in Figure 1, the new ISO Guide 33 will provide a normative reference to ISO Guide 34 and will cover the various types of RM uses, specified for certified and non-certified RMs. Consequently calibration will be included, which will eventually lead to the withdrawal of ISO Guide 32.
“In-house RMs”
Many reference materials, in particular those used for laboratory-internal quality control, are either prepared within the laboratory or by an organisation’s own “quality/metrology department” at the same location. Several requirements for these RMs may be somewhat less severe than for RMs, and in particular for CRMs, which have a wider range of intended uses and which are produced for worldwide distribution. Therefore, a separate guidance document was deemed to be required. The new ISO Guide 80 will offer approaches to produce in-house RMs which can be used in-house for quality control purposes. This will not include applications such as calibration or validation of trueness for measurement methods.
RMs for “qualitative analysis”
RMs for qualitative analysis are growing in number and in importance. So far, guidance on the production and use of RMs has been mainly focused on RMs/CRMs for quantitative measurements. For the authentication of food (e.g. wine, honey), the fight against doping in sports, the control of pesticides and other residues in foodstuff, the identification of microorganisms, these RMs are very important. In chemical analysis many requests are directed to the identification of a substance, rather than determining its concentration.
Based on the examples received so far it was concluded that three different types of methods are in use related to qualitative testing:
(A) quantitative methods interpreted qualitatively,
(B) qualitative methods,
(C) qualitative methods interpreted quantitatively.
In the detailed discussion which followed, it was decided that the future ISO Guide 79 should focus only on the purely qualitative methods (category B). The collected nominal property examples and the related information provided have demonstrated that all nominal testing fields need guidance concerning the estimation of the confidence level of qualitative tests. The essential question is how sure one can be about the identity of a material. In several areas, reliable and well-established practices are used to ensure the identity of a material with the required confidence level, information which the producer of a qualitative RM has to provide. Likewise a description ensuring the trackability (tracking of the origin) of the RM might be required. Therefore, Working Group 13 is now tasked with producing the first draft for a future ISO Guide 79 “Reference materials for qualitative analysis (testing of nominal properties)” for discussion at the next meeting. Among other aspects this draft should give guidance on the establishment of confidence levels for nominal testing materials.
The diversity of issues associated with drafting such a document has prompted ISO/REMCO to request assistance from contributors which are not REMCO delegates but have expertise in particular fields important for the development of this guide. To date, a few offers of assistance have been received, but in general the response has been very disappointing, and much more support in this area is required.
Metrological traceability
During the meeting this fundamental issue was again discussed at length, and for this reason ISO/REMCO has converted AHG2 into WG 15 “Metrological traceability”, with the Terms of Reference as follows:
- To compile an inventory of current documents and recommendations on the establishment of metrological traceability of measurement results.
- To derive, on the basis of the inventory, a viable and applicable interpretation of the current traceability definition of the VIM (JCGM 200:2008) for the production of reference materials.
- To draft a Technical Report (TR 16476) on the establishment of metrological traceability and its statement on CRM certificates and related communications with the working title “Reference materials—Establishing and expressing metrological traceability of quantity values assigned to reference materials”.
- To develop guidance on the establishment and expression of metrological traceability of (C)RM values, with a specific view to those CRM at the highest level of the traceability chain (CRM serving as primary measurement standards).
Accompanying documentation
ISO Guide 31 “Reference materials—Contents of certificates and labels”, aims to provide producers with a clear description of the minimum information requirements for documents accompanying reference materials. These requirements were discussed at the meeting, and the proposal for the revision is to focus on accompanying documentation and recommend that label requirements are determined by others.
Synopsis
Again, in 2010, the principal message from the meeting was to make use of effective communication via the various modern electronic media now available to assist the timely production of new and updated documentation, and to seek appropriate assistance outside of the committee for these important tasks.
Should you wish to contribute to the work of ISO/REMCO, please contact the Chair, detailing your specific area of expertise: Professor Dr Hendrik Emons, E-mail: [email protected]
Next meeting
The next ISO/REMCO meeting will be held in Delft, The Netherlands, on 11–15 July 2011.
References
- ISO Guide 30—terms and definitions used in connection with reference materials, (1992/Amd 1:2008). ISO, Geneva (2008).
- ISO Guide 31—reference materials––contents of certificates and labels. ISO, Geneva (2000).
- ISO Guide 33—uses of certified reference materials. ISO, Geneva (2000).
- ISO Guide 35—reference materials––general and statistical principles of certification, 3rd Edn. ISO, Geneva (2006).
- ISO Guide 34—general requirements for the competence of reference material producers, 2nd Edn. ISO, Geneva (2000).
- ISO 17025—general requirements for the competence of testing and calibration laboratories. ISO, Geneva (1999).
- ISO 17025—general requirements for the competence of testing and calibration laboratories, 2nd Edn. ISO, Geneva (2005).
- CIPM, Mutual recognition of national measurement standards and of calibration and measurement certificates issued by national metrology institutes. Sevres, France (October 1999).
- ISO Guide 32—calibration in analytical chemistry using certified reference materials. ISO, Geneva (1997).