Rodolfo J. Romañach
Department of Chemistry, University of Puerto Rico at Mayagüez
Editor’s summary
Sampling can be seen from many viewpoints: technical, economical, managerial… Here, sampling is described as a critical success factor in business cases, broadening the viewpoints presented above and below.
Introduction
Leading pharmaceutical companies continuously acquire technology to develop a quality product and bring it to market in the shortest possible time, realising that the growth and health of the company depends on new sales. They do not want the lack of new technology to stand in the way of competition for market opportunities. Leading companies are also committed to meeting the demands of their supply chain. Once a new product is approved, they want to supply their customers and always meet the expected delivery date. The 2020–2021 pandemic has emphasised the need for pharmaceutical manufacturing and timely delivery of products to counteract COVID-19 and provide medications for related conditions.1 Companies have invested heavily in Process Analytical Technology (PAT) to monitor and control processes, and in continuous manufacturing. They realise the need to consistently and rapidly monitor materials and interim products during production in which they increasingly rely on integrated, on-line analytics.2
To be able always to acquire representative samples, or representative PAT signals, is correctly viewed as one of the most challenging aspects in reliable process monitoring.2 Key examples are presented below in which proper sampling is a critical economic success factor in business cases.
Pharmaceutical sampling: lots of positive economic opportunities
Pharmaceutical sampling is carried out to serve various critical purposes:
- There are currently multiple efforts to eliminate manual sampling in the synthesis of small molecule drugs and in biotechnology-based products.2,3 This interest is especially evident with cell culture media where manual sampling could result in contamination.3 Automated sampling is seen as a way towards assured representative sampling.2 Automated sampling systems are being developed for the synthesis of Active Pharmaceutical Ingredients (API), where the acquired samples have to be prepared (e.g. removing particulate material) before injection into an on-line chromatographic system.2,4 Synthesis often involves sample extraction which, if performed manually, would be time consuming and impractical for long processes. Automated systems seek to eliminate the variability which could be introduced by different analysts, and avoid possible sample integrity problems.4 The goal is to integrate representative sample acquisition with subsequent preparation for injection into a chromatographic system, data processing and to make the results obtained available for process control.2,4These developments have obvious positive economic benefits and can readily be included and emphasised in business cases.
- Sampling is also performed to identify incoming raw materials.5 The current Good Manufacturing Practices (cGMP) and other regulations require that all raw materials be identified before use in a pharmaceutical process. The identification method is currently performed through handheld Raman or near infrared (NIR) spectrometers at many manufacturing sites. The business case is here a significant reduction of time needed for analysis. The handheld systems permit reliable identification of raw materials directly at the warehouse where materials are received. Thanks to handheld systems, it is no longer necessary to transfer the material to a local or remote laboratory. Handheld systems also facilitate digital transfer of the identification results to Laboratory Information Management Systems, reducing the risk of errors associated with manual entry of results—again with obvious economic benefits easily outlined in business cases.
- Very significant efforts have always been made to monitor the uniformity of powder blends.6 Pharmaceutical blends are usually constituted by several excipients and one or more APIs. Pharmaceutical regulations require that the uniformity of blends be evaluated before tablets are compressed. It is of considerable professional concern that sampling of such blends is still usually done through thief sampling, which is nothing but grab sampling, and multiple serious problems occur at this stage.6,7 Thief sampling requires interrupting the manufacturing process for several hours, and often requires special gowning and protection to reduce the exposure of personnel to potent drugs—all of which cause severe additional costs. Current good news, however, is that all of this can be avoided by judicious application of the Theory of Sampling (TOS).6,7TOS becomes a valued integral element in any business case in Pharma.
- Simultaneous sampling-and-analysis. In recent years, NIR and Raman spectroscopy have been used to monitor drug concentrations at the feed frame, immediately before tablets are compressed.8–10 The feed frame, and a stream sampler currently under development, are the main agents for meeting the Fundamental Sampling Principle (FSP) in which all parts of a moving lot must have the same opportunity of being sampled for analysis.11 NIR and Raman spectroscopic methods are essential parts of real-time monitoring and control approaches within the field of PAT. These methods are non-destructive, analyse the material in their native state and thereby eliminate the use of solvents in analyses. Wider implementation of PAT methods will reduce the use of solvents significantly, avoid operator exposure to potent drugs, and will further improve the uniformity of the tablets manufactured. However, as thief sampling still remains the main method for sampling powder blends; a stern call for caution has been made,6 which has considerable positive economic opportunities.
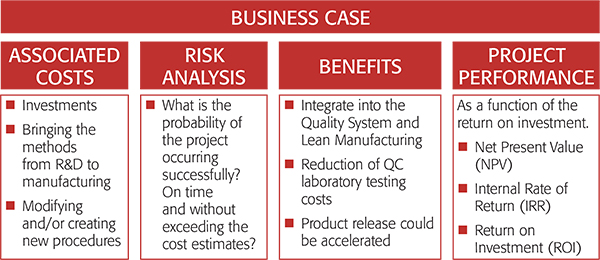
Key economic analysis tools for sampling.
Sampling in business cases
All the industrial applications of defensible representative sampling described above have on one or other occasion required preparation and approval as part of a business case. Investments in automation, PAT and continuous manufacturing require the approval of a business case by company management. The business case is how all new technology is presented in the company and corporation regimen, describes the investments needed, the likely economic benefits as well as plans for risk management and avoidance.12
The pharmaceutical industry presents multiple challenges for sampling of products which may be liquids, suspensions, tablets, small molecules or proteins. It is difficult to estimate the specific monetary gain potentials in this highly varied scenario. However, the Center for Structured Organic Particulate Systems, University of Puerto Rico at Mayagüez is currently developing a template to present business cases for new investments in PAT, sampling equipment and continuous manufacturing to pharmaceutical industry leaders.12 This novel template has provided new insights into the adoption of new technologies in the pharmaceutical industry, including sampling. A business case template will significantly add to the persuasion power of involving proper sampling wherever needed.
Acknowledgement
The author thanks the Economic Development Administration, award 01-79-14889 for the support of the current project on business cases.
References
- K.A. Moore, M. Lipsitch, J.M. Barry and M.T. Osterholm, COVID-19: the CIDRAP Viewpoint. University of Minnesota (2020). https://printabletemplates.com/cidrap-covid19-viewpoint/
- G.R. Lambertus, L.P. Webster, T.M. Braden, B.M. Campbell, J. McClary Groh, T.D. Maloney, P. Milenbaugh, R.D. Spencer, W.-m. Sun and M.D. Johnson, “Development of universal, automated sample acquisition, preparation, and delivery devices and methods for pharmaceutical applications”, Org. Process Res. Dev. 23(2), 189–210 (2019). https://doi.org/10.1021/acs.oprd.8b00280
- T.E. Matthews, B.N. Berry, J. Smelko, J. Moretto, B. Moore and K. Wiltberger, “Closed loop control of lactate concentration in mammalian cell culture by Raman spectroscopy leads to improved cell density, viability, and biopharmaceutical protein production”, Biotech. Bioeng. 113(11), 2416–2424 (2016). https://doi.org/10.1002/bit.26018
- D.C. Patel, Y.F. Lyu, J. Gandarilla and S. Doherty, “Unattended reaction monitoring using an automated microfluidic sampler and on-line liquid chromatography”, Anal. Chim. Acta 1004, 32–39 (2018). https://doi.org/10.1016/j.aca.2017.11.070
- T.E. Matthews, C. Coffman, D. Kolwyck, D. Hill and J.E. Dickens, “Enabling robust and rapid raw material identification and release by handheld Raman spectroscopy”, PDA J. Pharm. Sci. Technol. 73(4), 356–372 (2019). https://doi.org/10.5731/pdajpst.2018.009563
- K.H. Esbensen, A.D. Román-Ospino, A. Sanchez and R.J. Romañach, “Adequacy and verifiability of pharmaceutical mixtures and dose units by variographic analysis (Theory of Sampling) – A call for a regulatory paradigm shift”, Int. J. Pharm. 499, 156–174 (2016). https://doi.org/10.1016/j.ijpharm.2015.12.038
- C. Pinzon de la Rosa, V. Rodriguez, M.L. Hormaza et al., “TOS meets the NSF I-CORPS™ program”, in 8th World Conference on Sampling and Blending, Perth, Australia, 2017. Australian Institute of Mining and Metallurgy, pp. 351–354 (2017).
- N.O. Sierra-Vega, R.J. Romañach and R. Méndez, “Feed frame: The last processing step before the tablet compaction in pharmaceutical manufacturing”, Int. J. Pharm. 572, 118728 (2019). https://doi.org/10.1016/j.ijpharm.2019.118728
- N.O. Sierra-Vega, K.M. Karry, R.J. Romañach and R. Méndez, “Monitoring of high-load dose formulations based on co-processed and non co-processed excipients”, Int. J. Pharm. 606, 120910 (2021). https://doi.org/10.1016/j.ijpharm.2021.120910
- E.M. Hetrick, Z. Shi, L.E. Barnes, A.W. Garrett, R.G. Rupard, T.T. Kramer, T.M. Cooper, D.P. Myers and B.C. Castle, “Development of near infrared spectroscopy-based process monitoring methodology for pharmaceutical continuous manufacturing using an offline calibration approach”, Anal. Chem. 89(17), 9175–9183 (2017). https://doi.org/10.1021/acs.analchem.7b01907
- N.O. Sierra-Vega, R.J. Romañach and R. Méndez, “Real-time quantification of low-dose cohesive formulations within a sampling interface for flowing powders”, Int. J. Pharm. 588, 119726 (2020). https://doi.org/10.1016/j.ijpharm.2020.119726
- M.A. Fontalvo-Lascano, M.I. Méndez-Piñero and R.J. Romañach, “Development of a business case model for process analytical technology implementation in the pharmaceutical industry”, in Proceedings of the 5th NA International Conference on Industrial Engineering and Operations Management, Detroit, Michigan, 10–14 August, 2020. IEOM Society International, pp. 1144–1152 (2020).

Rodolfo J. Romañach
Dr Rodolfo Romañach is Professor of Chemistry at the University of Puerto Rico – Mayagüez Campus, and site leader for the Center for Structured Organic Particulate Systems. He worked in the pharmaceutical industry for over 12 years before joining the UPR Chemistry Department in 1999. He found his mission in training a new generation of pharmaceutical scientists capable of doing real time process measurements in the manufacturing area. He is presently continuing efforts to improve the teaching of chemometrics and further his understanding of the errors that affect real time process measurements—and what to do about all this.  0000-0001-7513-7261
0000-0001-7513-7261
[email protected]

