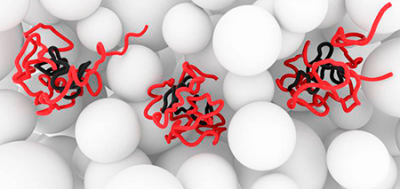
The protein α-synuclein plays an important role in Parkinson’s and other neurodegenerative diseases. Although a considerable amount is known about the structure of the protein within the Parkinson’s-typical amyloid deposits, nothing was known about its original state in the healthy cell up to now. Scientists from the Leibniz-Institut für Molekulare Pharmakologie (FMP) in Berlin have visualised the protein in healthy cells with the help of high-resolution nuclear magnetic resonance and electron paramagnetic resonance spectroscopic procedures. Surprisingly, they discovered an unstructured state. The new findings, which have appeared in Nature and Nature Communications, represent a milestone for research worldwide: It is now known that the structure of the protein changes dramatically over the course of the disease.
Neurodegenerative diseases such as Parkinson's, Alzheimer’s or Huntington’s have one thing in common: so-called amyloid aggregates are deposited in the brain. Amyloid is the umbrella term for protein fragments that are produced by the body and that ultimately lead to the demise of nerve cells. The protein α-synuclein is one of the main components of the amyloid aggregates and therefore plays a major role in the development of Parkinson’s disease. Much is known about the structural aspects of these aggregates. For example, it is known that α-synuclein has a very concrete structure, which means that it is based on a blueprint that follows a specific pattern. And, in contrast to this, it is known that the isolated, purified protein has no structure whatsoever.
However, up to now, it was not known what α-synuclein looks like inside a healthy cell. And pathological changes can only be fully explained if the original state of the protein is known. Researchers from the Leibniz-Institut für Molekulare Pharmakologie (FMP) in Berlin have succeeded in demonstrating, and visualising, α-synuclein in neuronal and non-neuronal cells. This was made possible by a combination of nuclear magnetic resonance spectroscopy (NMR) and electron paramagnetic resonance spectroscopy (EPR), two procedures that make it possible to characterise the structural configuration of a protein at atomic resolution.
“We discovered the unstructured state that the protein also has in the purified state”, explains Dr Philipp Selenko, Head of the In-cell NMR Spectroscopy research group. “This is actually rather surprising, because it was inconceivable up to now that such an unstructured state can survive at all in a cellular milieu.”
However, apparently cells can indeed deal with unstructured proteins. The images published in Nature show how the protein in the healthy cell protects the so-called NAC region from the penetration of foreign molecules. This central region plays a decisive role in the development of highly structured amyloid aggregates. Why the protective properties of the protein are lost in neurodegenerative diseases is one of the core questions with which research will be concerned in the future. “In the diseased state, this protein must change structurally to such an extent that the NAC region becomes accessible for other molecules, so that these regions can accumulate, start to grow and thus form the amyloid structures”, suspects Selenko.
The findings from Berlin provide the basis for an elucidation of these structural changes. The FMP researchers already have concrete plans for the coming months. With a few tricks, they will create artificially aged cells and introduce the amyloid protein and observe it using the same spectroscopic procedures. The age simulation is performed, because Parkinson’s and other neurodegenerative diseases are age-related diseases. Ultimately, the researchers want to construct a state that corresponds to the origin of the disease. “We hope to watch the protein as the protection of the NAC region is gradually lost and how it begins to form amyloid-like structures”, says Selenko.
In the study published in Nature Communications, the team of researchers had already made an exciting discovery in this respect. They had damaged the protein α-synuclein at several points in such a way as is normally the case in an aged cell. The protein was then introduced into a young, healthy cell. There, the researchers observed how the cell was able to repair the defects with amazing perfection in some regions, but not in others. The region that could not be repaired was the one that is immensely important for the function of the protein.
In the forthcoming study, the researchers want to gain a comprehensive insight into which defects cause the repair mechanisms to fail and thus prepare the ground for neurodegenerative processes. According to Philipp Selenko, this holds the key to at last finding the cause of the disease and thus one day intervening in the destructive process—with active substances that will then have to be developed on the basis of these findings. “With our discovery of the starting state of the protein, we have taken the decisive first step in this direction”, says Selenko.