John Hammond
Starna Scientific Limited, 52/54 Fowler Road, Hainault, Essex IG6 3UT, UK. E-mail: [email protected]
ISO/REMCO 40th meeting report
A synopsis of the above meeting is detailed below. The meeting was structured with plenary sessions at the start and finish, with appropriate Working Group meetings and discussions between these two.
Discussion topics
The plenary sessions in addition to agreeing an agenda and detailing the aims for the meeting, also included reports from the ISO/REMCO Chair and the ISO Secretariat, and introduced, and then discussed, the additional key topics of:
- how to increase the active membership of ISO/REMCO,
- how to improve the liaison coordination of ISO/REMCO and
- new opportunities to promote the work of ISO/REMCO using the Internet and social media.
Membership update and liaison coordination
The strong liaison relationships that ISO/REMCO has built up with international organisations over past years since its inception in 1975—for example, ISO/CASCO and the International Laboratory Accreditation Cooperation (ILAC)—again proved invaluable in 2017. It provided the necessary communication framework for input into the new and revised 17000 series standards. ISO 17034 was developed by ISO/CASCO in a joint working group with ISO/REMCO, and was published on 1 November 2016.1 ISO/REMCO also made inputs to the current revision of ISO/IEC 170252 to clarify the requirements for metrological traceability using certified reference materials and to include the use of reference materials, especially for assuring the quality of results.
As has been stated many times in this column, the requirement for “Quality” is a continuously moving and changing requirement and this is reflected in the later section of this article with the updates to the current implementation of ISO 17034 and ISO/IEC 17025 by the national accreditation bodies.
In relation to this, ISO/REMCO is currently providing input into the proposed revision of the International Vocabulary of Metrology (VIM) “VIM 4” under the direction of the BIPM JCGM Working Group (WG2), where specifically there is the requirement to update the definitions of what are both a “Reference Material” and a “Certified Reference Material”.
As currently defined in ISO 17034, a Certified Reference Material (CRM) is a reference material (RM), where in addition to the stated requirements for the RM, the metrological traceability of the certified value is stated, together with a value for the uncertainty of the property value, where by definition (and inclusion as a normative reference) these should be produced under ISO/IEC 17025.
Table 1 shows the requirements for ISO 17034.1
Table 1. Production requirements for RMs and CRMs.
General requirements | All RMs | CRMs | Applicable subclause |
Production planning | Required | Required | 7.2 |
Production control | Required | Required | 7.3 |
Material handling and storage | Required | Required | 7.4 |
Material processing | Required | Required | 7.5 |
Measurement procedures | Required | Required | 7.6 |
Measuring equipment | Required | Required | 7.7 |
Data integrity and evaluation | Required | Required | 7.8 |
Metrological traceability of certified values | Not required | Required | 7.9 |
Assessment of homogeneity | Required | Required | 7.10 |
Assessment and monitoring of stability | Required | Required | 7.11 |
Characterisation | Required, where values are to be assigned | Required | 7.12 |
Assignment of property values | Required, where values are to be assigned | Required | 7.13 |
Assignment of the uncertainties of the property values | Not required | Required for certified values | 7.13 |
RM documents and labels | Required | Required | 7.14 |
Distribution service | Required | Required | 7.15 |
Control of quality and technical records | Required | Required | 7.16 |
Management of non-conforming work | Required | Required | 7.17 |
Handling of complaints | Required | Required | 7.18 |
Once the dust has settled, after what will no doubt be a lively debate from many sides, be assured these changes to these fundamental definitions will be communicated via this column.
New opportunities for the promotion of the work of ISO/REMCO
In 2009, a hard-copy information booklet was published that gave an overview of the scope and objectives, the structure as well as the guidance documents being developed and maintained by the committee. Following the success of the booklet, the working group responsible (WG6) started to investigate other avenues for the promotion of the work of ISO/REMCO, and this has resulted in an information “booklet”, Introduction to ISO/REMCO, being made available in Portable Document Format (.pdf) in October 2017.
This document extensively uses hyperlinks to appropriate websites to maintain the document as up-to-date as possible, and where the latest version is shown by the date in the file name.3
At the meeting, there was also a presentation of the ISO/REMCO website currently in development. This website resides within the overall ISO environment and follows ISO-defined design and structure. However, these Technical Committee sites are defined to promote and share news, information etc., alongside the more structured ISO website. The development continues with the aim of making the website in 2018.
Work programme
In addition to these important discussion items, ISO/REMCO has an active work programme associated with reference materials, with its Guides and Technical Report programme. The principal activities for 2018 and beyond are as follows.
Guidance for the characterisation and the assessment of the homogeneity and stability of reference materials
As previously discussed, the new ISO Guide 35: “Reference Materials—Guidance for characterisation and assessment of homogeneity and stability” was published in September 2017, and is therefore available for purchase.4 It provides the essential primary reference guidance for the measurement of these fundamental requirements associated with reference materials, and thereby the essential control parameters to assist compliance to ISO 17034.
Guidance on the production of qualitative reference materials and the assignment of nominal properties
Following on from discussion during the meeting a request for approval for a new work item has been submitted to establish a guidance document for the production of qualitative reference materials, which has subsequently been accepted by the Technical Management Board (TMB) of ISO, and which will be discussed at the next meeting in Ottawa in July 2018.
Investigating the possibilities for new work items
Given that the natural revision cycle associated with the (new) standard ISO 17034 and the associated Guides has now entered the quiet post-release phase, after the publication of the new/revised documentation, then there is the opportunity to re-direct resources into potentially new areas of interest, by the formation of ad hoc groups. Tasked with reviewing new ideas, the ad hoc groups formed in 2016 gave feedback on the review work that had been performed in preparation for the development of terms of reference and possible new work item proposals for the groups.
Following on from an extensive review and discussion in the ad hoc group AHG4 “Development of guidance on the characterisation of pure chemical materials” Working Group meeting, it was decided that, unsurprisingly, the existing scope was too broad to be effectively covered by one topic. Therefore, AHG4 has been renamed to “Purity RMs for small organic molecules” and a new group has been created, AHG6 “Purity RMs for inorganic materials”.
Both groups have been tasked with searching and reviewing the existing documents relating to the appropriate reference materials, for discussion during the 41st ISO/REMCO Meeting (July 2018).
Ad hoc group 5 (AGH5) “Development of new approaches to the assessment of homogeneity and stability” focussed their efforts in searching available documentation on this topic, and a preliminary report was presented; the conclusion of which was principally that whilst there were a small number of new topics discussed, there was no major advancement in alternative procedures to those which have already been extensively revised and documented in the new ISO Guide 35. However, AHG5 will complete searches on reference material producer (RMP) websites and international organisations, such as the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) for new information on homogeneity and stability assessment approaches and will provide a recommendation to REMCO for further action at the 41st ISO/REMCO meeting.
At the meeting, there was also a request tabled with respect to the need for developing a guidance document for RMs in process analytical technologies (PAT) and for characterisation of RMs for multivariate properties, and this suggestion is being submitted to the ISO/REMCO membership for approval in 2018, for discussion in July.
Because of these changes, the structure of ISO/REMCO has been revised and is shown in Figure 1.
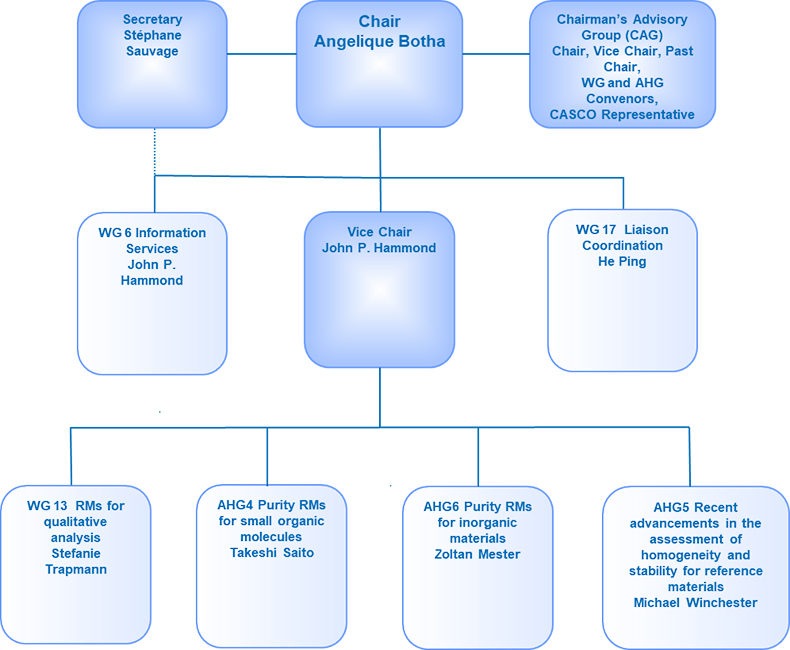
Figure 1. The revised structure of ISO/REMCO.
Next meeting
The 41st meeting of ISO/REMCO will be held in Ottawa, Canada from 10 to 13 July 2018.
New 17000 series standards: implementation update
In this fast-changing environment, catalysed by the new ISO 17034:2016 and ISO/IEC 17025:2017, an update is detailed below as to how the accreditation of these standards is being implemented by the national accreditation body (UKAS), from a UK perspective.
ISO 17034
The new ISO 170341 outlines the general requirements for the producers of RMs, including certified reference materials (CRMs). It has been both completely technically and structurally revised. For this reason, it is fully aligned with the new ISO/IEC 17025:2017. Also, not previously stated, but implicit in the transition of a “Guide” to a “Standard”, is the ability now for harmonisation within the member states of the European Union, and thereby the provision for Multilateral Recognition Agreements (MLAs) to be established.
Therefore, this paradigm shift from “Guide” to “Standard” can be likened to the similar transition that occurred from ISO Guide 25 to ISO/IEC 17025 many years ago. Many of these end-users, accredited to the new ISO/IEC 17025:2017 will be laboratories, where for the first time there are clear and unambiguous statements in the standard that “Certified values of certified reference materials from reference material producers (RMPs) conforming to ISO 17034 provide metrological traceability” and “RMPs fulfilling the requirements of ISO 17034 are considered to be competent”.
Unsurprisingly, given the time-frame agreed within ILAC, national accreditation bodies have already trained assessors, agreed requirements, established programmes and have initiated the transition process to ISO 17034. From personal experience, there are already organisations now accredited to ISO 17034:2016. Fortunately, in most, if not all, national accreditation bodies the number of RMPs is relatively small, and therefore the logistics of the process is correspondingly simple.
ISO/IEC 17025
Given the chronological separation of the publication dates of these two new standards, by approximately twelve months, national accreditation body implementation of the transition programme for ISO/IEC 170252 also reflects this separation. One would anticipate that an organisation transitioned to ISO 17034:2016 in early 2018, would be expected to be transitioned to the associated ISO/IEC 17025:2017 again, one year later, in early 2019.
However, as previously stated, the mandated implementation period by ILAC for the national accreditation bodies, is three years, and “…the clock is ticking”. If one considers, for example that there are approximately 1600 calibration and testing laboratories accredited to ISO/IEC 17025 by the United Kingdom Accreditation Service (UKAS) in the UK, then the logistics of this process could surely be described as “challenging”!
So, in summary, in 2018:
- For RMPs, there is now an international standard, one normative reference and three ISO Guides that support the production and certification of RMs to ensure that the quality of the RMs meets the requirements of the ISO 17000 accredited laboratories; and for the users of Certified Reference Materials in those environments, at long last there, is an ISO 17000 standard combination which will provide the confidence in manufacturing using ISO 17034, and the certified value assignment, associated expanded uncertainty budget and traceability, via ISO/IEC 17025.
- As has often be stated in this column when discussing the purchase of CRMs, the phrase caveat emptor, (definition: “the principle that the buyer alone is responsible for checking the quality and suitability of goods before a purchase is made”) applies; but now, if a RMP is accredited to both these new ISO 17000 standards, surely “the first quality question on the list” has been unreservedly answered in the affirmative?
References
- ISO 17034, General Requirements for the Competence of Reference Material Producers. International Organization for Standardization (ISO), Geneva, Switzerland (2016).
- ISO/IEC 17025, General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO), Geneva, Switzerland (2017).
- Introduction to REMCO. International Organization for Standardization (ISO), Geneva, Switzerland. This document is available at https://www.iso.org/committee/55002.html by using the “Quick Link” to access the working area, then the Public Information folder and finally the 09 REMCO materials folder.
- ISO Guide 35, Reference Materials—Guidance for Characterisation and Assessment of Homogeneity and Stability. International Organization for Standardization (ISO), Geneva, Switzerland (2017).



