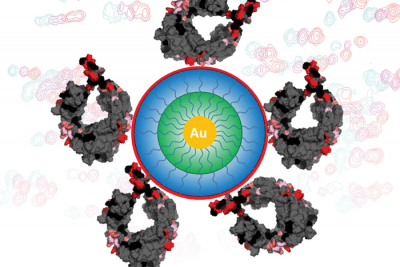
The process of purifying biopharmaceutical drugs remains a costly and time-consuming challenge. A deeper understanding of how unwanted elements within bio-manufactured proteins bind to the molecules developed to remove them could help researchers make purity processes more efficient, more complex and increasingly scalable.
A team led by Steven Cramer (Rensselaer Polytechnic Institute) have used nuclear magnetic resonance (NMR) spectroscopy to explore the fundamentals of how different molecules interact with various surfaces during the purification process.
“This is part of a very big effort to understand the fundamentals of how these molecules interact with surfaces”, said Cramer. “Our group is trying to ramp up the intellectual level of this kind of analysis in a variety of ways.”
Working with Merck Pharmaceuticals and Bio-Rad Laboratories, and using the NMR core facility in the Rensselaer Center for Biotechnology and Interdisciplinary Studies, the team looked at the Fc part of an IgG1 antibody and how ligands interact with that section of the antibody protein in particular. IgG1 antibodies are used in a large majority of biopharmaceutical drugs, meaning a deeper understanding of how their components interact with various molecules and ligands could have widespread implications.
Now, the team has taken its exploration even deeper by adding nanoparticles to the surface of various proteins in order to see exactly where the ligands are binding.
“With this approach, we can then also look at some of the subtle interactions that are happening”, Cramer said. “Sometimes these ligands come together and form these clusters of ligands, and these clusters actually drastically change the behaviour.”
This work, Cramer said, could lead to the development of new and improved materials and ligands. It also could help researchers develop more nuanced and specific ways of separating out unwanted molecules that are very similar to another type of molecule that needs to remain. All of these advancements could improve the drug purification process, making it more efficient and effective.