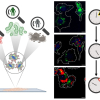
A new technique developed by researchers at Lawrence Livermore National Lab promises to improve accuracy and lower costs of real-time assessment of kidney function, reports an article published in Journal of Biomedical Optics. The paper explores the use of multimodal autofluorescence and light scattering to evaluate functional changes in the kidneys after ischemic injury. Conditions including accumulated arterial plaque or blood clots restrict the flow of oxygen and glucose to organs, and prolonged periods of such ischemia can compromise function.
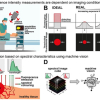
While other current work in this area uses expensive multiphoton and laser-based techniques, the authors reduced expenses by switching to camera-based imaging. Currently, there is no real-time tool to measure the degree of ischemic injury incurred in tissue or to predict the return of its function. The inability to decisively determine tissue functional status runs two great risks: that dysfunctional tissue may be transplanted, increasing the morbidity and mortality of the patient; and that much-needed functional kidney tissue may be discarded.
In their study, Rajesh Raman of Lawrence Livermore National Lab and co-authors Christopher Pivetti and Christoph Troppmann of the University of California Davis, Rajendra Ramsamooj of California Northstate University, and Stavros Demos of Lawrence Livermore acquired autofluorescence images of kidneys in vivo under 355 nm, 325 nm and 266 nm illumination. Light-scattering images were collected at the excitation wavelengths while using a relatively narrow band light centred at 500 nm.
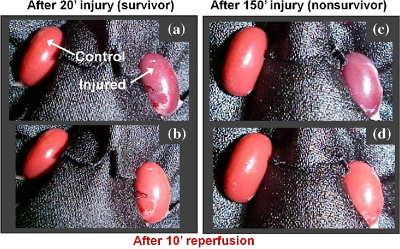
The images were simultaneously recorded using a multimodal optical imaging system. The recorded signals were then analysed to obtain time constants, which were correlated to kidney dysfunction as determined by a subsequent survival study and histopathological analysis. Analysis of the light-scattering and autofluorescence images suggests that variations in tissue microstructure, fluorophore emission and blood absorption spectral characteristics, combined with vascular response, contribute to the behaviour of the recorded signals. These are used to obtain tissue functional information and enable the ability to predict post-transplant kidney function. This information can also be applied to the prediction of kidney failure when visual observation cannot, almost immediately following an injury.