Daniel B. Thomasa and Cushla M. McGoverinb
aInstitute of Natural and Mathematical Sciences, Massey University, Auckland, New Zealand.
E-mail: [email protected]
bThe Dodd Walls Centre for Photonic and Quantum Technologies, Department of Physics, University of Auckland, Auckland, New Zealand
Introduction
Pigments have important roles in the physiologies and ecologies of a diverse range of organisms, from unicellular algae to fungi, plants and animals (see, for example, Figure 1). Biological pigmentation is used for communication and camouflage, as well as maintaining health, for producing and circulating respiratory gases, and many other functions. Pigmentation research often encourages researchers with a background in biology to become familiar with analytical instrumentation more commonly used by dedicated chemists. Ultraviolet/visible spectroscopy (UV/vis) and high-performance liquid chromatography (HPLC) are staples of biochrome research, where the former is non-destructive and the latter is routinely used for chemical identification. Raman spectroscopy combines both of these advantages into a single technique, but is relatively under-utilised in pigment biology research. Raman spectroscopy provides information about functional groups in a molecule and can be used to identify biological pigments. Raman spectroscopy has the potential for wide use in pigmentation research alongside HPLC and UV/vis. The aim of this short review article is to show how Raman spectroscopy is being used in biological pigment research, beginning with studies of carotenoid pigmentation.

Carotenoids
The characteristic colours of canaries, marigolds, carrots and butterflies are all examples of the yellow through orange to red colours conferred by carotenoids. Carotenoids contain 40 carbon atoms and are divided into two groups on the basis of whether oxygen is present or not, xanthophylls and carotenes, respectively. Carotenoids are composed of a conjugated double bond chain which may or may not be terminated by ring structures. These shared structural traits result in three carotenoid characteristic peaks within Raman spectra: C=C stretching at 1500–1535 cm–1, C–C stretching at 1145–1165 cm–1 and C–CH3 deformation at 1000–1010 cm–1. The structural differences between carotenoids in the terminating rings, polyene side chains and polyene length alter the Raman shift of these three dominant peaks, and introduce smaller peaks which are carotenoid specific. It is therefore possible using these peaks and their positions thereof to use Raman spectroscopy for the identification of carotenoids. This approach was recently used to non-destructively characterise the most abundant carotenoid in the feathers of 36 bird species.1 However, these spectra were all collected from a similar matrix, keratin of the bird feather, and therefore matrix effects did not introduce a significant source of spectral variance that would perturb carotenoid characterisation when comparing the feather spectra. Raman spectra of carotenoids are environment dependent; therefore, spectra from biological tissues should not be compared to extracted carotenoid spectra for the purposes of identifying carotenoids. For example, mango tissue (Mangifera indica) contains a single form of carotenoid, b-carotene; the C=C stretching mode peak in the Raman spectrum of mango tissue occurs at 1529 cm–1, however, in the Raman spectrum recorded from the extracted b-carotene this peak is at 1515 cm–1.2 The differences between extract and matrix-bound carotenoid spectra is, in part, a consequence of shape changes induced by the matrix. Changes in the planarity of the polyene chain result in peak shifting, particularly the C=C stretching band. In addition, the matrix may provide environments of different hydrophobicity, which will affect the extent to which the highly hydrophobic carotenoids interact with the surrounding matrix, e.g. the red carotenoid lycopene is present in tomatoes as microcrystalline aggregates due to the aqueous nature of the matrix.
Unsubstituted linear polyacetylenes
Bright biochromes are also common in marine environments, e.g. the red skeleton of Corallium sea fan corals. Sea fans and many other octocorals (i.e. corals with eight-fold symmetry) can also have yellow, pink and purple skeletons, and these exclusively-marine animals have a long history of being harvested for use in jewellery and other ornaments. Despite the centuries of tradable value, the pigmentary basis for octocoral skeletons has remained largely unknown. Relatively recent studies of pigmented octocoral skeletons with Raman spectroscopy sought an end to the mystery and recovered spectra with major peaks around 1100–1120 cm–1 and 1500–1520 cm–1, but the spectra did not have a strong peak at 1000 cm–1. These spectra suggested that octocoral pigments may be “psittacofulvins”, a group of pigments that had previously only been reported from parrot feathers.3,4
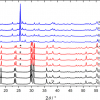
Parrots are unusual among birds in that the red, orange and yellow colours of their plumage are not the result of carotenoid pigmentation. Instead, parrots achieve brightly coloured plumage with a group of polyunsaturated linear aldehydes (polyenals), pigment compounds for which the structure was first predicted with Raman spectroscopy.5 As with carotenoid molecules, the conjugated carbon backbones of psittacofulvins give rise to strong C=C (e.g. 1500–1520 cm–1) and C–C (e.g. 1100–1120 cm–1) peaks in Raman spectra. The backbones of psittacofulvins are not methylated as they are in carotenoids though, and the C–CH3 deformation mode (e.g. 1000 cm–1) that helps identify a carotenoid is absent in spectra from parrot feathers (see Figure 2). In contrast, spectra from octocorals have a minor peak around 1004–1020 cm–1 attributed to C–CH3 rocking. Although a fully resolved structure for the octocoral pigment is yet to be reported, the presence of a C–CH3 rocking peak in the former is strong evidence that octocorals and parrot feathers have distinct pigments. Raman spectroscopy has shown that octocoral and parrot pigments have similar architecture, an unsubstituted linear polyacetylene backbone,4 but that “psittacofulvins” are still a parrot-specific plumage pigment.

Tetrapyrroles
Birds achieve vivid colouration with a suite of chemically-distinct pigments, including the tetrapyrrole pigments that colour the surfaces of eggshells. Brown eggshells are coloured with protoporphyrin IX (e.g. chicken eggs), the precursor macrocycle for haeme, and the classic “eggshell blue” and blue-green shell colours are conferred with biliverdin, a linear tetrapyrrole pigment broken down from haeme. Blue-green and brown pigments help to camouflage eggs and can aid parents in distinguishing their own eggs from an egg laid by a brood parasite (e.g. cuckoo). While most pigmented eggshells are brown, blue-green or a mixture thereof, some comparatively rare eggshells are pink, orange or bright green.6 The bright green eggshells of an Elegant Crested Tinamou (Eudromia elegans) were recently studied with Raman spectroscopy.7 Seven peaks in Raman spectra from the Elegant Crested Tinamou eggshells both identified a tetrapyrrole pigment and distinguished biliverdin from protoporphyrin (1619, 1588, 1467, 1295, 1248, 1174 and 970 cm–1). The two most intense peaks from the pigment were at 1295 cm–1 and 1248 cm–1 which identified the C=C and C–N stretching of the pyrrole subunits. Hence, Raman spectroscopy showed that biliverdin is responsible for a wide blue to green colour gamut in eggshells. Moreover, the ability to identify biliverdin in eggshells without destroying the specimen is a useful application for studying ancient remains (i.e. zooarchaeology).
Moa are extinct ostrich-like birds that inhabited New Zealand until the 15th century. Eggshell fragments from upland moa (Megalapteryx didinus) are olive-green or faint blue and suggest that biological pigments can be preserved in eggshells over extended time scales. Ancient eggshell fragments with pigmentation are rare and destructive analyses are not always viable. Instead, faint blue moa eggshell fragments were analysed with non-destructive Raman spectroscopy, confirming the presence of biliverdin.7 Beyond presence or absence data, information about pigment concentration could provide valuable insight into the ecology of these extinct birds. Pigment concentrations could potentially be gained from Raman spectral peak ratios calibrated with concentration data from HPLC. The strongest peak in a Raman spectrum of a pigmented eggshell is attributed to the symmetric stretching of the carbonate anion in calcium carbonate.7 The relative intensities of carbonate and pigment peaks are expected to change with concentration, providing a non-destructive method for quantifying pigment levels.
Unresolved pigments
Colourful animals held in museum collections are excellent resources for studying evolutionary patterns in biological pigmentation. Raman spectroscopy is ideally suited for surveying the pigments of museum specimens as the technique requires no specialised sample preparation and analyses are rapid (i.e. specimen handling time is short). Known pigments in biological tissues can be quickly identified (e.g. carotenoids in feathers), and there is the potential for new pigments to be discovered. For example, an unusual yellow feather pigment was recently identified in the feathers of some penguin species.8 The solubility, light absorption and fluorescence properties of the penguin pigment were revealed to be substantially different from any known plumage pigment. More information about the chemical identity of the penguin pigment was sought with Raman spectroscopy.
Functional groups in the penguin pigment identified with Raman spectroscopy were consistent with a nitrogen-bearing heterocyclic ring.9 A distinct peak at 1466 cm–1 was attributed to the stretching of bonds in a lactam ring (i.e. cyclic amide) and peaks at 1285 cm–1 and 1351 cm–1 were attributed to C–N stretching and C–C stretching. Nitrogen heterocycles occur in several types of pigment including pterins, porphyrins and linear tetrapyrroles. The yellow penguin pigment and the tetrapyrrole pigment bilirubin have broadly similar Raman spectra, except that the penguin pigment has a peak at 1577 cm–1 with the highest relative intensity.9 The Raman spectrum from the penguin pigment does not otherwise match any other published spectrum. The penguin pigment has proven challenging to extract from the feather for mass spectrometric analyses and structural elucidation. Resolving the structure of the yellow penguin pigment will provide deep insight into the function and evolutionary origins of the unusual yellow pigment.
Anthocyanins
Raman spectroscopy is widely used to confirm the presence of a known compound in a particular sample. However, when the sensitivity and chemical specificity of standard Raman spectroscopy are insufficient for an analytical problem, the surface enhancement effect may be needed. In surface-enhanced Raman spectroscopy (SERS) specific molecular vibrations are enhanced when the molecule is adsorbed to, or in proximity of, a coinage metal surface with nanometric roughness (e.g. silver or gold nanoparticles, silver or gold nano-patterned surfaces). Surface-enhanced Raman spectroscopy has recently been used to characterise plant sources of anthocyanins (red, purple and blue plant pigments).10 Anthocyanins have a long history of use as dyes particularly for textiles. SERS spectra were recorded from plants pigmented with anthocyanins (bilberry, elderberry, purple corn, sumac and hollyhock) and extracts from aged and unaged dyed textiles. Spectral peaks characteristic to anthocyanins were observed in the spectra recorded from each plant species sample.
Moreover, pigments extracted from wool fibres (as little as 0.5 mg) produced SERS spectra with anthocyanin-identifying peaks.10 However, even the sensitive SERS spectra were insufficient for identifying specific anthocyanins or plant sources in the wool spectra. This experiment indicated that it is possible to identify the presence of anthocyanins in textiles even after the colour has faded; a result that has implications for the analysis of these dyes in historical textiles.
Conclusion
Raman spectroscopy is used in biological pigment research as a method for quickly confirming the presence of a known biochrome. Researchers have also used the technique to identify particular pigments beyond the generalised pigment class, to investigate the chemical identities of unknown pigments, and although not fully explored here, to measure pigment concentrations. Raman spectroscopy is ideal for studying pigments in situ (i.e. without extraction from biological tissues) as there is no specialised sample preparation required. We feel that Raman spectroscopy could be more widely used in biology research, and we hope that this brief review encourages pigment researchers to explore the value of this technique for their own study systems.
References
- D.B. Thomas, K.J. McGraw, H.F. James and O. Madden, “Non-destructive descriptions of carotenoids in feathers using Raman spectroscopy”, Anal. Methods 6, 1301 (2014). doi: http://dx.doi.org/10.1039/C3AY41870G
- V.E. de Oliveira, H.V. Castro, H.G.M. Edwards and L.F.C. de Oliveira, “Carotenes and carotenoids in natural biological samples: a Raman spectroscopic analysis”, J. Raman Spectrosc. 41, 642 (2010). doi: http://dx.doi.org/10.1002/jrs.2493
- L.F. Maia, B.G. Fleury, B.G. Lages, J.P. Barbosa, A.C. Pinto, H.V. Castro, V.E. de Oliveira, H.G.M. Edwards and L.F.C. de Oliveira, “Identification of reddish pigments in octocorals by Raman spectroscopy”, J. Raman Spectrosc. 42, 653 (2011). doi: http://dx.doi.org/10.1002/jrs.2758
- R.F. Fernandes, L.F. Maia, M.R.C. Couri, L.A.S. Costa and L.F.C. de Oliveira, “Identification of reddish pigments in octocorals by Raman spectroscopy”, Spectrochim. Acta A 134, 434 (2015). doi: http://dx.doi.org/10.1016/j.saa.2014.06.022
- M. Veronelli, G. Zerbi and R. Stradi, “In situ resonance Raman spectra of carotenoids in bird’s feathers”, J. Raman Spectrosc. 26, 683 (1995). doi: http://dx.doi.org/10.1002/jrs.1250260815
- M.E. Hauber and J. Bates, The Book of Eggs: A Lifesize Guide to the Eggs of Six Hundred of the World’s Bird Species. University of Chicago Press, Chicago, IL, USA (2014). doi: http://dx.doi.org/10.7208/chicago/9780226057811.001.0001
- D.B. Thomas, M.E. Hauber, D. Hanley, G.I.N. Waterhouse, S. Fraser and K.C. Gordon, “Analysing avian eggshell pigments with Raman spectroscopy”, J. Exp. Biol. 218, 2670 (2015). doi: http://dx.doi.org/10.1242/jeb.124917
- K.J. McGraw, M.B. Toomey, P.M. Nolan, N.I. Morehouse, M. Massaro and P. Jouventin, “A description of unique fluorescent yellow pigments in penguin feathers”, Pigment Cell Res. 20, 301 (2007). doi: http://dx.doi.org/10.1111/j.1600-0749.2007.00386.x
- D.B. Thomas, C.M. McGoverin, K.J. McGraw, H.F. James and O. Madden, “Vibrational spectroscopic analyses of unique yellow feather pigments (spheniscins) in penguins”, J. Roy. Soc. Interface 10, 20121065 (2013). doi: http://dx.doi.org/10.1098/rsif.2012.1065
- C. Zaffino, S. Bruni, B. Russo, R. Pilu, C. Lago and G.M. Colonna, “Identification of anthocyanins in plant sources and textiles by surface-enhanced Raman spectroscopy (SERS)”, J. Raman Spectrosc. in press (2015). doi: http://dx.doi.org/10.1002/jrs.4786