Jan Novotný, Karel Novotný, David Prochazka, Aleš Hrdlička and Jozef Kaiser
Laboratory of Laser Spectroscopy, Institute of Physical Engineering, FME & CEITEC BUT – Central European Institute of Technology, Brno University of Technology, Technická 2896/2, 616 69 Brno, Czech Republic. E-mail: [email protected]
Introduction
Laser-Induced breakdown spectroscopy (LIBS),1 a relatively young technique of atomic emission spectroscopy, uses as its excitation source a focused laser pulse and this effective combination brings to the field of elemental analysis a number of significant advantages.
Although the first LIBS analysis happened shortly after the construction of the first laser in 1962, development at a much larger scale occurred at the beginning of the 1980s mainly due to the production of modern powerful Nd:YAG lasers and charge coupled device (CCD) detectors.
Today, LIBS is the subject of ever increasing interest due to its speed, relatively simple instrumentation setup, no demands for a sample preparation and the possibility to determine most of the periodic table elements, along with other attributes. LIBS can be used to perform a spatial resolved analysis, thus is capable of being used for depth profiling and surface mapping. Surface mapping and creating so-called “chemical maps”,2 (or “chemical images” of the analysed sample) are presented here as an example of LIBS applications.
Physical principle
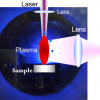
LIBS utilises the spectral analysis of an optical emission of a plasma plume in order to determine the elemental composition of the analysed material. The analysis starts with the focusing of a high-power laser pulse onto the sample surface with the spot diameter generally between units to hundreds of microns. High irradiance of the order of GW-TW cm–2 melts down and evaporates a small amount of any material in any state of matter. Particles are excited by the means of multiphoton absorption, chemical bonds are broken and the sample is atomised. Free electrons gain further energy by collision with other electrons or ions and by inverse Bremsstrahlung. Ionisation spreads rapidly, resulting in the creation of a plasma with an electron density approximately 1017—1020 cm–3, temperature of 103—104 K and size up to few millimetres — depending on the laser pulse energy and ambient pressure. Initial plasma expansion speed can be 105 ms–1 order of magnitude and generates a characteristic clearly audible shock wave.
From the very first moments of plasma existence the emission spectrum is dominated by the continuous background caused by Bremsstrahlung. When the laser pulse ends, the plasma begins to cool down, the background level is gradually attenuated and atomic emission lines of the elements present become clearly visible. The best time window for capturing the emission spectra is generally from hundreds of ns to a few tens of µs as measured from the laser pulse triggering. After this time the intensity of atomic lines decrease and molecular bands starts to appear.
The optical spectral range from the 200 nm to 900 nm contains atomic emission lines of most of the chemical elements, thus it is generally possible to perform a complete qualitative analysis in a few seconds with just a single laser shot. It is necessary to note that LIBS cannot directly determine chemical compounds as they have been decomposed in the plasma.
Performing a quantitative analysis for the determination of concentrates requires one to carry out a calibration. Measuring a series of standards with known concentration or using internal standardsation are the most common techniques. A technique known as calibration-free LIBS3 can also be also used. In this case concentrations are determined by theoretical calculations of plasma parameters (such as temperature and electron density) without the need of any calibration data. Due to the fluctuations of the laser-induced plasma, applying some standardisation technique is advisable.
Limits of detection are generally in the range of 1–100 ppm depending on the type of sample and element of interest. More details can be found in References 1 and 4.
Instrumentation
A typical LIBS setup (see Figure 1) consists of several basic components. The pulsed laser generator, a laser focusing lens to induce a plasma onto the sample surface, a collecting lens to collect the plasma radiation and the spectrometer with the detector to capture dispersed emission spectra. The total system, namely the detector exposure with respect to the laser trigger, is usually synchronised by a pulse generator.

Even though the assembly of the typical LIBS setup is relatively simple, high demands are placed on the operating parameters of the individual components. Development of the LIBS instrument and its applications are highly dependent on the progress of developments in the fields of pulsed lasers, spectrometers and detectors of optical radiation. Pulsed lasers capable of high irradiation (> GWcm–2) are necessary in order to induce a plasma on most samples. Optically pumped Q-switched solid state (Nd:YAG, Ti:Sa) or fibre lasers are currently widely used for their robustness and price-to-performance ratio. Wavelength ranges from the infrared (IR) to UV, pulse duration from ns to fs, pulse and energies from µJ to hundreds of mJ.
Spectrometers of high throughput and resolution are necessary. Although echelle types deserve special mention for their exceptional combination of high resolution with wide spectral range (covering usually the whole LIBS range from 200 nm to 900 nm) Czerny–Turner configurations are still preferred for their sensitivity.
Due to the plasma emission dynamics, the key parameter of the detector is its time resolution. A fast electronic shutter that is able to limit the exposure time to units of µs can greatly improve the signal-to-background ratio of the atomic emission lines. It is clear that other parameters such as high sensitivity, quantum efficiency, low dark current and high dynamic range are also of high importance. Intensified charge-coupled device (ICCD) detectors with a multi-channel plate (MCP) have proved to be one of the best solutions for LIBS applications, although sometimes the utilisation of lower cost and more compact electron multiplying CCDs (EMCCDs) can be beneficial.
On the other hand the low cost of a very specialised LIBS setup can be built up utilising a pulsed microchip laser and suitable optical filter with photodiode for detection. But it is important to note that the performance of such a setup would be rather limited.
Methods and applications
A standard table-top single-pulse LIBS setup can be modified to enhance its LIBS spectroscopic capabilities or to accommodate a LIBS technique for a new application.5
Double-pulsed LIBS,6 a combination of LIBS plus laser-induced fluorescence,7 applying nanoparticles onto a sample surface8 or just changing the type and pressure of ambient atmosphere9 can significantly increase the signal-to-background ratio, thus improving the limits of detection for trace elements. High magnification microscopy objectives focusing the Gaussian laser pulse to a µm sized spot enable a µLIBS analysis with high-spatial resolution. Liquids and samples dissolved in liquids can be quickly analysed by means of liquid LIBS targeting the laser pulse directly into the stream.10 LIBS can also analyse gases and aerosols, and has the possibility to analyse a quantity of single nanoparticle size.11
LIBS setups can be made mobile in order to perform an in situ analysis with shape and size similar to a modern hand-held XRF (X-ray fluorescence) detector. Since the laser pulse and plasma radiation can be easily transmitted through the ambient air or optical fibres, the analysis can be undertaken remotely at a distance usually from a few units up to a few tens of metres.12 Remote LIBS utilises optical fibres with a hand-held probe, while in stand-off LIBS the laser pulse is focused directly onto the sample by means of a telescope. Successful analysis at a distance of more than 100 m has been reported using a stand-off LIBS setup.13
Applications of the LIBS technique as an analytical tool for fast chemical analysis are focused, inter alia, mainly in the fields of the steel industry — chemical analysis of metal alloys and rapid online analysis of melted steel, detection of toxic elements in the food industry, environmental diagnostics, chemical analysis in archaeology and cultural heritage, determination of mineral contents in geology, trace analysis forensic analysis and detection of explosives. The rapidness of LIBS analysis and the possibility to measure samples in situ and remotely enable measurements to be carried out in places and situations inaccessible to other more conventional techniques; examples of such locations are the environment of nuclear stations and extraterrestrial research (CHEMCAM in the Curiosity Rover by NASA).14
LIBS can be very effectively combined with Raman spectroscopy to obtain more detailed information about a sample’s chemical composition15 or with computed tomography (CT) in order to reconstruct a 3D map of chemical element distribution.
Chemical imaging
Chemical imaging is today of great interest in many fields of materials analysis. It can, for instance, provide information about the surface distribution of the individual chemical elements within the analysed area in a very clear visual form. Utilising LIBS for this purpose brings a number of benefits. Analysis is relatively fast, does not need any sample preparation and the resulting chemical map contains a spectroscopic trace of all the chemical elements present. The spatial resolution of LIBS can be as high as tens or even units of microns, depending on the laser energy profile and parameters of the laser focusing lens.
Depending on the required resolution and sample area covered, it may be necessary to carry out from hundreds to tens of thousands measurements. Automated measurements therefore can speed up the process and save a significant amount of time.
The team at the Laboratory of Laser Spectroscopy at Brno University of Technology (Brno, Czech Republic) has been working with the LIBS method for more than 15 years with special focus on biological, geological and archaeological samples. Chemical mapping2 is widely used in the laboratory to detect and visualise the distribution of the desired chemical element on a sample surface. The central component of laboratory table-top LIBS setup is the interaction chamber, which has been developed by the laboratory research team in order to meet the requirements of the LIBS method and its modifications. The chamber contains the motorised manipulator, and using the control software it is possible to carry out automated 2D mapping.

For the chemical mapping that is described in the next section, the following Double-Pulse LIBS configuration has been used (see Figure 2):
- pulsed Nd:YAG laser Solar LQ-529 (pulse length 8 ns, pulse energy up to 280 mJ, wavelength 532 nm) as a primary laser for inducing the plasma on the sample surface,
- pulsed Nd:YAG laser Quantel Brilliant B (pulse length 8 ns, pulse energy up to 800 mJ, wavelength 1064/532 nm), as a secondary laser for reheating the plasma,
- echelle spectrograph Andor Mechelle 5000 (spectral range 200–975 nm),
- ICCD detector Andor iStar 734 with Image Intensifier tubes (Micro Channel Plate, MCP), internal pulse generator (nanosecond time resolution) and
- pulse generator Stanford DG535 for synchronisation of individual components.
Experimental
Examples of maps of selected elements are shown in Figures 3 and 4. An area of 8 mm × 8 mm was analysed on the surface of a sperrylite (a platinum arsenide mineral) and a cut chalcopyrite (copper iron sulfide mineral) stone (left side of Figure 3). The spatial resolution was 100 µm, the diameter of the crater was approximately 50 µm and the depth of the crater about 6 µm. The goal was to visualise the surface distribution of the elements present, such as Pt, Pb and Ni.


Double-pulsed LIBS was used in order to obtain the best possible spectra by single measurement. The primary pulse at 532 nm had energy of 30 mJ, after a time interval of 1.5 ns the secondary pulse reheated the plasma plume with a pulse energy of 100 mJ and wavelength 1064 nm.
The map was created by an automated measurement of a matrix containing 6400 spots, thus the map data includes the same number of spectra as there was no accumulation (one spectra per measurement spot). Due to the wide spectral range of the echelle spectrometer (UV-vis-NIR) every measurement contains evidence of all the chemical elements present. By defining the wavelength of characteristic atomic emission lines one can easily set the map to visualise the distribution of any desired chemical element, see, for example, Figures 3 and 4.
A similar measurement was made for another sample of chalcopyrite (see Figure 5) in order to locate the galena veins. Since galena is a compound of lead and sulfur (PbS) the element of interest was lead which has its atomic emission line at 405.708 nm. In this particular case, a much larger area of 25 × 25 mm2 was analysed on the surface of the cut (left side of Figure 5).

As with the previous case of sperrylite mapping, the measurement was done using the orthogonal double-pulse LIBS technique using the same laser and detector parameters. The spatial resolution was 100 µm and the crater diameter approximately 50 µm. The chemical map was built up using more than 60,000 measurements. Signal intensity in Figure 5 reflects the spatial distribution of lead on the sample cross-section. The intensity colour scale in arbitrary units can be used for semi-quantitative estimation of the lead content or after signal calibration even for quantitative determination.
As has been mentioned above, LIBS can be effectively combined with computed tomography in order to gain more information about the sample and its properties. CT can provide structure information and a 3D model of the sample, in which materials of different physical properties are distinguished and LIBS can identify the chemical elements of these materials. A result of this combined analysis can be seen in Figure 6. The 3D distribution of galena veins inside the sample is revealed and this can be used for estimation of the lead amount within the whole sample.

Conclusions
Spatially resolved materials analysis is highly demanded in many fields of modern material science and industry. LIBS is able to perform such an analysis. Its spatial resolution is limited mainly by the laser crater diameter but can be as small as a few units of microns. The other benefits, such as speed, no need for sample preparation, possibility to measure in situ and even stand-off, make LIBS an effective tool for visualisation of a chemical element distribution on the sample surface. Some of the chemical maps shown in this article recorded from the recently analysed geology samples illustrate this capability.
Despite the fact that there are still some drawbacks, e.g. slightly problematic quantitative analysis and the matrix effect, LIBS has become a highly-respected technique, complementary to other well-known techniques of material analysis, such as atomic absorption spectroscopy, X-ray fluorescence, inductively coupled plasma mass spectroscopy, gas chromatography–mass spectrometry (GC-MS) etc.
The LIBS technique is not only studied and used within many research institutes and universities, but instrumentation is being increasingly developed commercially due to the ever-growing need for real-world and industrial applications. There are a number of companies now with a product portfolio that includes LIBS technology. The authors of this article are developing LIBS and its instrumentation with the close cooperation of their University and, the first spin-off of their research centre, the AtomTrace Company (www.atomtrace.com).
Acknowledgements
The authors would like to acknowledge the project “Development of an interaction chamber for laser-induced breakdown spectroscopy (LIBS)” (TA02011272) by the Technology Agency of the Czech Republic (TACˇR) and project “CEITEC—Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) from the European Regional Development Fund. Jan Novotný acknowledges the project CZ.1.07/2.3.00/30.0005: “Support of interdisciplinary excellence research teams establishment at BUT”.
References
- R. Noll, Laser-Induced Breakdown Spectroscopy: Fundamentals and Applications. Springer (2012). doi: http://dx.doi.org/10.1007/978-3-642-20668-9
- V. Piñón, M.P. Mateo and G. Nicolas, “Laser-induced breakdown spectroscopy for chemical mapping of materials”, Appl. Spectrosc. Rev. 48, 357–383 (2013).
doi: http://dx.doi.org/10.1080/05704928.2012.717569 - E. Tognoni, G. Cristoforetti, S. Legnaioli and V. Palleschi, “Calibration-free laser-induced breakdown spectroscopy: state of the art”, Spectrochim. Acta Part B At. Spectrosc. 65, 1–14 (2010).
- D.W. Hahn and N. Omenetto, “Laser-induced breakdown spectroscopy (LIBS), part I: review of basic diagnostics and plasma-particle interactions: still-challenging issues within the analytical plasma community”, Appl. Spectrosc. 64, 335–66 (2010). doi: http://dx.doi.org/10.1366/000370210793561691
- D.W. Hahn and N. Omenetto, “Laser-induced breakdown spectroscopy (LIBS), part II: review of instrumental and methodological approaches to material analysis and applications to different fields”, Appl. Spectrosc. 66, 347–419 (2012). doi: http://dx.doi.org/10.1366/11-06574
- V.I. Babushok, F.C. De Lucia, J.L. Gottfried, C. A. Munson and A.W. Miziolek, “Double pulse laser ablation and plasma: Laser induced breakdown spectroscopy signal enhancement”, Spectrochim. Acta Part B At. Spectrosc. 61, 999–1014 (2006). doi: http://dx.doi.org/10.1016/j.sab.2006.09.003
- H.H. Telle, D.C. Beddows, G.W. Morris and O. Samek, “Sensitive and selective spectrochemical analysis of metallic samples: the combination of laser-induced breakdown spectroscopy and laser-induced fluorescence spectroscopy”, Spectrochim. Acta Part B At. Spectrosc. 56, 947–960 (2001). doi: http://dx.doi.org/10.1016/S0584-8547(01)00190-2
- A.De Giacomo, R. Gaudiuso, C. Koral, M. Dell’Aglio and O. De Pascale, “Nanoparticle enhanced laser induced breakdown spectroscopy: effect of nanoparticle deposited on sample surface on laser ablation and plasma emission”, Spectrochim. Acta Part B At. Spectrosc. 98, 19–27 (2014). doi: http://dx.doi.org/10.1016/j.sab.2014.05.010
- G. Asimellis, S. Hamilton, A. Giannoudakos and M. Kompitsas, “Controlled inert gas environment for enhanced chlorine and fluorine detection in the visible and near-infrared by laser-induced breakdown spectroscopy”, Spectrochim. Acta Part B At. Spectrosc. 60, 1132–1139 (2005). doi: http://dx.doi.org/10.1016/j.sab.2005.05.035
- P. Porˇízka, D. Prochazka, Z. Pilát, L. Krajcarová, J. Kaiser, R. Malina, et al., “Application of laser-induced breakdown spectroscopy to the analysis of algal biomass for industrial biotechnology”, Spectrochim. Acta Part B At. Spectrosc. 74–75, 169–176 (2012). doi:
http://dx.doi.org/10.1016/j.sab.2012.06.014 - F.J. Fortes, A. Fernández-Bravo and J. Javier Laserna, “Chemical characterization of single micro- and nano-particles by optical catapulting–optical trapping–laser-induced breakdown spectroscopy”, Spectrochim. Acta Part B At. Spectrosc. 100, 78–85 (2014). doi: http://dx.doi.org/10.1016/j.sab.2014.08.023
- F.J. Fortes and J.J. Laserna, “The development of fieldable laser-induced breakdown spectrometer: no limits on the horizon”, Spectrochim. Acta Part B At. Spectrosc. 65, 975–990 (2010). doi: http://dx.doi.org/10.1016/j.sab.2010.11.009
- S. Palanco and J. Laserna, “Remote sensing instrument for solid samples based on open-path atomic emission spectrometry”, Rev. Sci. Instrum. 75, 2068 (2004). doi: http://dx.doi.org/10.1063/1.1753675
- A.K. Knight, N.L. Scherbarth, D.A. Cremers and M.J. Ferris, “Characterization of laser-induced breakdown spectroscopy (LIBS) for application to space exploration”, Appl. Spectrosc. 54, 331–340 (2000). doi: http://dx.doi.org/10.1366/0003702001949591
- S.K. Sharma, A.K. Misra, P.G. Lucey and R.C.F. Lentz, “A combined remote Raman and LIBS instrument for characterizing minerals with 532 nm laser excitation”, Spectrochim. Acta A. Mol. Biomol. Spectrosc. 73, 468–476 (2009). doi: http://dx.doi.org/10.1016/j.saa.2008.08.005