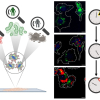
Improved analytical tools are urgently required to identify degradation and failure mechanisms in Li-ion batteries. However, understanding and ultimately avoiding these harmful mechanisms requires continuous tracking of complex electrochemical processes in different battery components. Now a team led by the NanoPhotonics Centre in the Cavendish Laboratory at the University of Cambridge has shown a way to monitor the chemistry of liquid electrolytes during battery cycling by Raman spectroscopy.
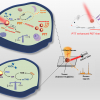
They periodically extract tiny liquid electrolyte samples from a working Li-ion battery into an optical fibre with a micro-sized hole down its centre. They then use the fibre’s light confinement to interrogate sub-microlitre samples by Raman spectroscopy. This approach enables the team to monitor the chemistry of industry-standard carbonate-based liquid electrolytes during electrochemical cycling in Li-ion batteries with a state-of-the-art high-energy-density cathode.
The team’s spectroscopic measurements reveal significant changes in the carbonate solvents and electrolyte additives during charging and discharging, allowing them to track how lithium-ions repeatably move across the battery.
“Rather than just probing a single material, real-time techniques provide a vital understanding of how these various materials function cooperatively, and crucially within working batteries,” enthuses co-investigator Professor Dame Clare Grey.
The new methodology contributes to understanding better the limitations of Li-ion batteries and paves the way for studies of degradation mechanisms in different electrochemical energy storage systems. The multidisciplinary work was carried out within the UK-wide Faraday Institution, in close collaboration with the Cambridge Chemistry Department, the Institute of Manufacturing, and the Max Planck Institute for the Science of Light in Erlangen, Germany.