Thanks in part to screening technologies like colonoscopy; colon cancer is often detected in its earliest stages. However, the procedure can be expensive and unpleasant. Patients must drink large quantities of laxatives the night before and undergo sedation or anaesthesia during the procedure itself. Current health guidelines in the USA say that adults should have a colonoscopy every ten years beginning at age 50—and sooner or more frequently if other risk factors exist—but US Centers for Disease Control and Prevention (CDC) statistics say that only 65% of American adults actually follow these guidelines. That is why many researchers are searching for less invasive alternatives to the colonoscopy that could induce encourage more people to get tested.
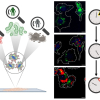
In a step towards this goal, researchers from the BC Cancer Agency and the University of British Columbia have identified differences in the blood of people with precancerous polyps compared to people without such polyps. Their results could eventually lead to a cheaper and less invasive initial screening test for colon cancer. The team collected blood plasma samples from three groups of people: 23 who had adenomatous (precancerous) polyps, 21 who had verified colon cancer and 25 healthy volunteers. They mixed each plasma sample with silver nanoparticles and then analysed the mixtures with surface-enhanced Raman spectroscopy (SERS). Their results are published in Biomedical Optics Express.
In previous work, the team’s collaborators at Fujian Normal University had shown a difference in the Raman spectra of the blood plasma of people with colon cancer compared to individuals who were cancer-free. This new study demonstrated that the test can also be used to identify people with precancerous polyps—a finding that makes it potentially more useful for a screening test, since it may be able to identify cancer risk before the disease actually develops.
A blood test that could detect precancerous polyps would be an improvement over other non-invasive colon cancer screening mechanisms currently available. For instance, the faecal occult blood test is an at-home kit that looks for evidence of microscopic traces of blood in stool samples, a potential sign of cancer. However, many early-stage colon cancers are asymptomatic and do not bleed, leaving them undetectable by this measure.
The initial results are promising, but the procedure is still in the research phase and must go through additional lab tests and clinical trials before actually being implemented. For instance, although the team identified significant spectral differences between the blood samples, they have not yet determined the exact biomolecular sources for these differences. “We are planning new research to identify these responsible molecules,” said Zeng.
A blood test for colon cancer will never replace colonoscopy: once polyps are identified, a colonoscopy is still the best way to locate them so that they can be removed. However, a blood test could be used as a first line of defence to identify patients with warning signs of cancer; these high-risk patients could then be referred for a colonoscopy for further investigation.