For the first time, researchers have detected protease activity with surface-enhanced Raman spectroscopy (SERS) performed using a tiny waveguide. The work paves the way to real-time, label-free lab-on-a-chip protease monitoring, which could offer a high-throughput approach to screen for new drugs that inhibit proteases involved in disease.
Proteases break down the peptide bonds that hold proteins together. They are important drug targets because of their involvement in many diseases, including cancer, Alzheimer’s disease and arthritis.
Nina Turk from the IMEC research centre at Ghent University in Belgium will present the new research at the all-virtual OSA Frontiers in Optics and Laser Science APS/DLS (FiO + LS) conference (14–17 September 2020).
“We hope that our interdisciplinary approach can one day enable fast and efficient discovery of new drugs for a variety of protease-linked diseases, thus improving lives of millions of patients around the world,” said Turk.
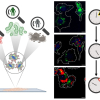
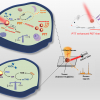
SERS uses a metal surface with nanoscale roughness to enhance weak signals produced when light interacts with a sample. Because of its high sensitivity, the spectroscopy technique can detect analytes in extremely small volumes. Although SERS has been used for sensitive and selective detection of proteases, this has only been demonstrated using a bulky Raman microscopy setup. Recently, nanoplasmonic slot waveguides have emerged as a new way to efficiently excite and collect SERS signals. These waveguides consist of two rails that form a small gap through which light can be guided. Coating the inside of the gap with gold nanostructures can be used to produce the SERS effect. Because of their small size, waveguides can be incorporated onto lab-on-a-chip devices, allowing simultaneous measurement of many analytes for high-throughput drug discovery.
To see if these nanoplasmonic slot waveguides would be useful for SERS detection of proteases, the researchers fabricated a waveguide and designed an experiment for detecting the trypsin protease. They created a specific peptide substrate for trypsin that binds to the gold nanostructure. When the trypsin peptide cleaves to the substrate, part of the substrate diffuses away, creating a detectable reduction in intensity for the SERS spectrum.
Their experiment revealed a 70 % decrease in SERS intensity after one hour of trypsin incubation, showing that nanoplasmonic slot waveguides could be used to detect trypsin. The researchers are now working to expand their platform so that it can detect the activity of two or more proteases simultaneously. The work was the collaboration between Ghent University-imec and the Flemish Institute for Biotechnology (VIB) under the supervision of Professors Roel Baets and Kris Gevaert.