Iain A. Larmour, Jennifer P.E.D. Gray and Steven E.J. Bell
Innovative Molecular Materials Group, School of Chemistry and Chemical Engineering, Queen’s University Belfast, Belfast, BT9 5AG, UK
Introduction
Interest in Raman spectroscopy as an analytical technique that can be applied in a wide variety of fields continues to increase. The main reason for this interest is that no special sample preparation is required. However, the Raman signal is typically very weak, with only one in every 106–108 photons being scattered. This has driven the development of several enhancement techniques, e.g. Resonance Raman (RR), Surface Enhanced Raman Spectroscopy (SERS) and Surface Enhanced Resonance Raman Spectroscopy (SERRS), which can be used for dilute samples.
In order to be enhanced, the target species must either have an electronic absorption at the laser excitation wavelength or be attracted towards a structured metallic substrate where the molecular vibrations can be coupled to the surface plasmons. Clearly, this means that these enhancing techniques are not universal.
One technique for enhancing the signal from dilute solutions that should be universal is solvent removal, which has the effect of increasing the concentration of the solute. When the concentration of the solution is increased, more solute molecules are encountered by the probe laser beam, which increases the overall signal. To a first approximation the absolute signal will increase linearly with the concentration. This means, for example, that if a 2 μL droplet (diameter of 1.56 mm) evaporates to 100 μm diameter the concentration and therefore the signal intensity will increase by >3000 ×. Continual removal of solvent down to a 10 μm diameter droplet increases the concentration by a factor >3 × 106 ×.
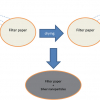
Although the potential signal enhancements are attractive and there are several well-known methods for solvent removal, each of them has drawbacks which severely limit their range of applicability. For example, simple drying on a surface is not straightforward. Consider drops of coffee accidentally spilled on a table. If left to evaporate they will form “coffee-ring” stains on the table, this is general for all solution drops placed on a standard flat surface and is caused by the pinning of the three-phase contact line of the drop that stops it contracting radially. This effect is coupled to internal flows within the evaporating droplet that cause the solute to be transported to the periphery, which enhances the pinning effect and eventually leads to formation of a ring deposit at the edge of the pinned droplet.
If a deposited droplet could retain a near perfect spherical shape as solvent was removed the solute should then become confined to a single solid deposit at the central position of the deposited droplet, rather than a ring. This can be achieved with a suitable, highly water repellent surface.