Timed to coincide with World Cancer Day on 4 February, Diamond Light Source are releasing a short video to mark a major advance in brain tumour diagnosis made by a research team from UK institutions using infrared spectroscopy at Diamond. This will enable non-invasive diagnoses of primary brain tumours (gliomas) and accelerate results as they will be available in real-time from a read out showing the kinds of mutations present in the gliomas of their patient. This could then help doctors make timely surgical decisions to deliver personalised medicine.
Gliomas constitute the vast majority of primary brain tumours and unfortunately, patients diagnosed with gliomas have poor clinical prognoses. Recently, a UK wide team of researchers used Diamond to study the protein IDH1, which is present in all gliomas. The researchers developed a method based on infrared radiation (IR) to determine if the IDH1 had a specific mutation: a mutated version of the IDH1 gene, if present in a glioma, is associated with a better clinical outcome for the patient. The current way to determine whether a glioma has the mutation is to take a biopsy, which can be dangerous and is invasive. A further complication is that several different biopsies may have to be taken across the entire tumour as the mutations are often not evenly distributed. Standard procedure is for the biopsy to be processed and stained in a lab for visible analysis, which means it takes longer to make a diagnosis.
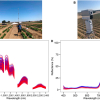
The research team used Diamond’s B22 beamline [Multimode InfraRed Imaging And Microspectroscopy (MIRIAM)] and other vibrational spectroscopy methods, in particular Fourier-transform infrared (FT-IR) spectroscopy. The research team studied the wild-type protein IDH1 and compared it to the mutant. They discovered that the mutant and the wild-type proteins indeed had different infrared spectral signatures, which could be reliably differentiated. They confirmed these results on biopsy sections from 79 glioma patients with both sensitivity and specificity significantly above 80 %.
This finding means that in the future, it might be possible for surgeons to use FT-IR analysis to get a real-time read out of the kinds of mutations present in the gliomas of their patients. This could then help them make timely medical decisions about the delivery of personalised medicine.
The research study also investigated whether blood serum could be analysed to give clinical information on gliomas. They wanted to know if they could measure any changes in the blood of patients that was linked to the mutation in the IDH1 enzyme. For this, blood serum samples from an additional 72 patients were collected. The research team discovered a method for processing the blood sample and accurately determining which variant of the IDH1 protein a patient had 69 % of the time.
This exciting finding provides clinicians with a relatively non-invasive way to better understand the brain tumours of individual patients. This gives them important information to administer more personalised treatments and decide on the kind of surgical intervention that might be needed.
Synchrotron IR is ideal for spectroscopy in exploratory research like this and allows high quality data even at the spatial scale of a cell in tissues. This combination of IR spectroscopy and microscopy allows researchers to look at smaller sections of human tissues with superior spectral sensitivity necessary for global analysis and refinement of the method.
Gianfelice Cinque, principal beamline scientist at Diamond, adds “Synchrotron based FT-IR has proven to be key in proof-of-concept biomedical studies. On this basis, it becomes easier to adapt IR microspectroscopy methods to lab instruments and focus only on the molecular differences that are important and were validated by synchrotron FTIR at Diamond, for example.”
“Our research gas further demonstrated the possible patient benefit that can be achieved from clinical spectroscopy. We are now working to translate these research advances into the clinic so we can positively affect the lives of patients worldwide”, explains lead author, Matthew Baker from University of Strathclyde and ClinSpec Diagnostics Ltd.