Just as blood pressure informs heart health, intracranial pressure (ICP) helps indicate brain health. ICP sensing is the burgeoning focus of Jana Kainerstorfer’s biomedical optics lab at Carnegie Mellon University. Her team is working to modernise ICP sensing approaches, which historically have been invasive and risky. Their non-invasive alternatives will ease risk of infection, pain and medical expenses, as well as present new monitoring capabilities for patients with an array of brain injuries and conditions, from stroke to hydrocephalus.
Investigating pressure levels in the brain is a laborious task for health professionals and hasn’t progressed much since the 1960s. Current practice involves drilling a hole into a patient’s skull and placing a probe inside for continuous monitoring of ICP levels. It comes with the risk of infection and damaging the brain itself, and while valuable data to have, ICP measurement is reserved only for the most critical of situations.
“At the core of it, what we’ve done is build a sensor alternative that doesn’t require drilling a hole into the patient’s head”, said Kainerstorfer, associate professor of biomedical engineering. “We recently published two papers that explore the use of optical sensors on the forehead for non-invasive ICP monitoring, using near infrared spectroscopy and diffuse correlation spectroscopy. Both approaches represent huge strides in improving the patient experience and providing better tools to monitor pressure levels in the brain, which can be a key variable in both diagnosis and treatment decisions.”
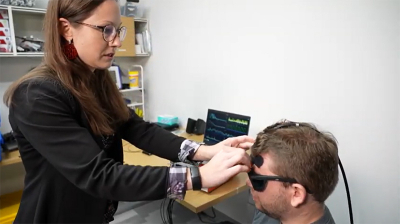
The first body of work, in collaboration with Matthew Smith, an associate professor of biomedical engineering at CMU, demonstrates that non-invasive near infrared spectroscopy can be used for ICP monitoring. The process involves small, customised sensors that are placed on the head to measure light that has interacted with the brain. Every time the heart beats, it produces a pulse in the brain, and the sensors measure haemodynamic blood volume/blood flow changes. Then, a machine learning algorithm is applied to determine ICP.
“Our aim is to create a portable and user-friendly tool to measure pressure changes in the brain”, explained Kainerstorfer. “We don’t believe that fancy hardware is needed to measure blood flow or haemoglobin concentration, and right now, we don’t have a good handle on how much brain pressure even fluctuates. There are other non-invasive methods that are out there, but what sets us apart is that we're using a sensor that can be made wearable and offers continuous monitoring.”
Kainerstorfer’s group also partnered with UPMC Children’s Hospital of Pittsburgh, in collaboration with Dr Michael McDowell, to conduct a clinical study utilising more specialised sensors and hardware to measure ICP, via non-invasive diffuse correlation spectroscopy. Fifteen patients from UPMC’s paediatric intensive care unit (ICU) participated, and their results were published in the Journal of Neurosurgery (doi.org/jpk4).
As part of the study, a probe with optic fibres was placed on the forehead of the paediatric patients who already had invasive ICP monitors to measure blood flow changes and quantify ICP and to compare the non-invasively acquired data to the invasively acquired data. The results of the non-invasive model were compared with those of invasive monitoring and found to be very similar.
“In this study, the patients had an invasive sensor placed in their brain, and it served as our ground truth”, elaborated Kainerstorfer. “We worked alongside outstanding collaborators and were encouraged by the results we saw, which indicated that a non-invasive approach could yield accurate ICP measurement. It still requires very specialised hardware and expertise to interpret the data, but it’s much better than drilling a hole in the head.”