Aoife C. Power,a, James Chapmanb,
James Chapmanb, and Daniel Cozzolinoc,*,
and Daniel Cozzolinoc,*,
aAgri-Chemistry Group, School of Medical and Applied Sciences, Central Queensland University (CQU), Bruce Highway, North Rockhampton, Queensland, 4701, Australia
bSchool of Science, RMIT University, GPO Box 2476, Melbourne, Victoria 3001, Australia
cAgri-Chemistry Group, School of Medical and Applied Sciences, Central Queensland University (CQU), Bruce Highway, North Rockhampton, Queensland, 4701, Australia, and School of Science, RMIT University, GPO Box 2476, Melbourne, Victoria 3001, Australia. E-mail: [email protected]
Introduction
New developments in the field of optics and electronics have resulted in an increasing number of new applications of spectroscopic analysis, which is further augmented by the introduction of portable and compact spectrophotometers.1–3 This miniaturised instrumentation allows analysts to explore unique practices in a diverse of fields including anthropology and forensics.4–11
Bone and bone materials are complex structures comprising of minerals (approximately 55–65 wt %), organic materials (approximately 25–35 wt %) and approximately 10 wt % water.3 The mineral component of bone consists of carbonated hydroxyapatite particles embedded in the organic matrix, a combination of collagen and non-collagenous proteins.3 The ratio of these inorganic and organic components will differ depending on the type, origin and function of the bone. Moreover, the environment of the subject will also influence the bone’s makeup, with numerous factors impacting, for example the host’s diet.3
The application of near infrared (NIR) spectroscopy for the discrimination of bone materials—either pulverised (bone meal) or as whole (bone fragments), in the study of archaeological materials with a primary focus on the conservation of delicate materials and as a forensic tool in art preservation—has been evaluated in a number of studies.4–11 More recently, the technique has been applied to a wide range of palaeontological studies, which has opened up an exciting opportunity for palaeontologists to employ non-destructive analytical techniques combined with chemometrics.4–11 This study aims to evaluate the capability of a portable NIR instrument to classify and identify the origin of skull bones from a number of different animal species (mammalian, avian and reptile).

Samples, spectra collection and analysis
Skull bone samples were sourced from different animal species belonging to the collection of skulls stored at the Science lab (CQU, Rockhampton, Queensland, Australia). Their origin was recorded as per the label in the box and they were stored at room temperature (26 °C ± 5). The NIR spectra of the samples were obtained from measurements at three random positions on the skull with a MicroNIR OnSite (Viavi, Santa Rosa, CA, USA) in the spectral range of 950–1650 nm. Whole samples were scanned and the resulting spectra analysed using principal component analysis (PCA) and partial least squares discriminant analysis regression (PLS-DA). More details about the methodology and samples can be found in a recent article.3
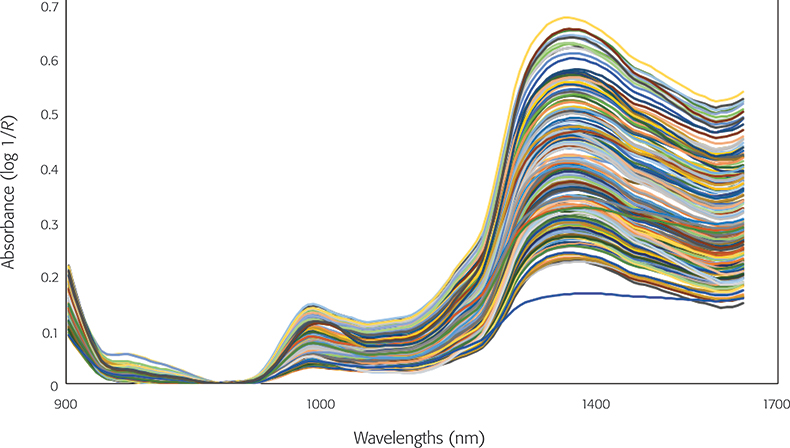
Figure 1. Near infrared spectra of skull samples.
Results and discussion
The NIR spectra of skull bones samples from each of the animal classes analysed in the region between 950 nm and 1650 nm are shown in Figure 1. The NIR spectra show variation in the intensity of some of the wavelengths. In particular, in absorption bands around 982 nm, which are related to combination vibrations of H–O–H bend and O–H stretch of water, and around 1186 nm with C–H bond vibrations.3,12 Differences between samples were also observed around 1400 nm associated with O–H bonds (1470 nm).3,12 Absorption bands near the O–H bonds were reported by other authors to be associated with the degradation of hydroxyapatite in external regions of bones and considered a very useful indicator of diagenetic processes in the bone.3 Figure 2 shows the PCA score plot for the classification of avian and mammalian skull bones samples. It has been observed that PC1 accounted for 77 % of the total variance while PC2 explained a further 21 % of the variance, demonstrating that NIR spectroscopy was capable of differentiating between the two animal classes. The loadings (not shown) highlighted the influence of wavelengths at 1007 nm, 1180 nm and 1447 nm associated with the stretching vibrations of the carbonated hydroxyapatite matrix of the bone, as described in the raw spectra.3
The classification rates based on the NIR spectra and PLS-DA analysis were 96 % and 81 % for the correct classification of skull bone samples as avian and mammalian, respectively. Overall, a 91 % correct classification rate was obtained for the classification of skull samples according to the class (mammalian and avian) which is equivalent or better than the classification rates reported in similar studies.3
The classification results obtained in this study infer that differences in the chemical composition of the bones might be responsible for the observed differences in the NIR spectra and thus in the classification results obtained. Differences in metabolism, nutrition and environment effects (sunlight degradation, season) might explain the observed differences between the skull bones from different families and species.3 However, this information was not available at the time of the analysis.
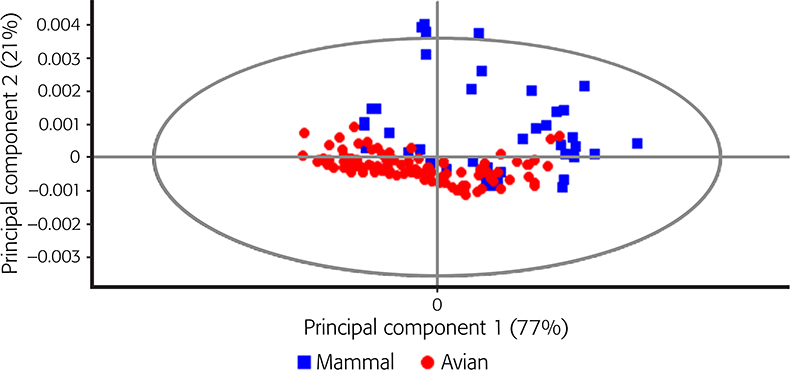
Figure 2. Principal score plot for the classification of avian and mammalian skull bones samples based on NIR reflectance spectra.
There is a wealth of information that can be obtained from vibrational spectroscopic examination of fossils and bones, ranging from taxonomic identification of enigmatic microfossils, fossil preservation mechanisms, diagenetic and thermal alteration pathways and histories of fossils, to chemical composition. Moreover, the results of this study indicated that the requirement to test large numbers of samples is not a prerequisite to validate and confirm the ability of NIR spectroscopy to differentiate/class bones of different species. Therefore, the results of this study have added to the state of the current knowledge in the application of NIR spectroscopy to analyse bones from a diverse range of animal species.
Given the non-specificity of the technique, these positive preliminary results indicate that this method of analysis has the potential to identify any animal bone sample. The non-invasive nature of this analysis ensures the quality of the sample is preserved. This contrasts favourably with traditional methodologies, which are expensive, time consuming and often require highly specialised operators and instrumentation.3,12 Therefore, the rapid nature, lack of consumables and sample preparation required results in a far more time- and cost-effective analysis per sample.3
Conclusion
This study demonstrates the potential of NIR spectroscopy coupled with chemometric data processing as a means of rapid, non-destructive classification of skull bone samples. It is apparent that this approach can readily distinguish between various animal classes and species of mammals and birds. The study highlighted the potential usefulness of the technique in the field as more accessible instruments appear in the market. The authors envision that further optimisation of this technique could lead to significant advances in the field of anthropology, archaeology and forensics. Potential applications include identification of bone fragments or even items fabricated from animal bone, such as ornamental figures or other artefacts made with bone fragments.
Acknowledgements
The supply of the skull bones by Central Queensland University is acknowledged.
References
- R.M. Correia, E. Domingos, W.M. Cao, B.R.F. Araujo, S. Sena, L.U. Pinheiro, A.M. Fontes, L.F.M. Aquino, E.C. Ferreira, P.R. Filgueiras and W. Romao, “Portable near infrared spectroscopy applied to quality control of Brazilian coffee”, Talanta 176, 26–33 (2018). doi: https://doi.org/10.1016/j.talanta.2017.07.094
- W.R. de Araujo, T.M.G. Cardoso, R.G. da Rocha, M.H.P. Santana, R.A.A. Munoz, E.M. Richter, T.R.L.C. Paixao and W.K.T. Coltro, “Portable analytical platforms for forensic chemistry: A review”, Anal. Chim. Acta 1034, 1–21 (2018). doi: https://doi.org/10.1016/j.aca.2018.06.014
- A.C. Power, J. Chapman, S. Chandra, J.J. Roberts and D. Cozzolino, “Illuminating the flesh of bone identification – An application of near infrared spectroscopy”, Vibr. Spectrosc. 98, 64–68 (2018). doi: https://doi.org/10.1016/j.vibspec.2018.07.011
- M. Buckley, S.W. Kansa, S. Howard, S. Campbell, J. Thomas-Oates and M. Collins, “Distinguishing between archaeological sheep and goat bones using a single collagen peptide”, J. Archaeol. Sci. 37, 13–20 (2010). doi: https://doi.org/10.1016/j.jas.2009.08.020
- V. Chavagnac, J. Milton, D. Green, J. Breuer, O. Bruguier, D. Jacob, T. Jong, G. Kamenov, J. Le Huray and Y. Liu, “Towards the development of a fossil bone geochemical standard: an inter-laboratory study”, Anal. Chim. Acta 599, 177–190 (2007). doi: https://doi.org/10.1016/j.aca.2007.08.015
- M. De la Haba, J.F. Pierna, O. Fumière, A. Garrido-Varo, J. Guerrero, D. Pérez-Marín, P. Dardenne and V. Baeten, “Discrimination of fish bones from other animal bones in the sedimented fraction of compound feeds by near infrared microscopy”, J. Near Infrared Spectrosc. 15, 81–88 (2007). doi: https://doi.org/10.1255/jnirs.688
- M.J. De la Haba, A. Garrido-Varo, D.C. Pérez-Marín and J.-E. Guerrero, “Near infrared spectroscopy calibrations for quantifying the animal species in processed animal proteins” J. Near Infrared Spectrosc. 17, 109–118 (2009). doi: https://doi.org/10.1255/jnirs.838
- B. De la Roza-Delgado, A. Soldado, A. Martínez-Fernández, F. Vicente, A. Garrido-Varo, D. Pérez-Marín, M. De la Haba and J. Guerrero-Ginel, “Application of near-infrared microscopy (NIRM) for the detection of meat and bone meals in animal feeds: A tool for food and feed safety”, Food Chem. 105, 1164–1170 (2007). doi: https://doi.org/10.1016/j.foodchem.2007.02.041
- I. Murray, L.S. Aucott and I.H. Pike, “Use of discriminant analysis on visible and near infrared reflectance spectra to detect adulteration of fishmeal with meat and bone meal”, J. Near Infrared Spectrosc. 9, 297–311 (2001). doi: https://doi.org/10.1255/jnirs.315
- M.M. Cascant, S. Rubio, G. Gallello, A. Pastor, S. Garrigues and M. De la Guardia, “Burned bones forensic investigations employing near infrared spectroscopy”, Vibr. Spectrosc. 90, 21–30 (2017). doi: https://doi.org/10.1016/j.vibspec.2017.02.005
- A.O. Marshall, C.P. Marshall and A. Smith, “Vibrational spectroscopy of fossils”, Palaeontology 58, 201–211 (2015). doi: https://doi.org/10.1111/pala.12144
- J. Workman and L. Weyer, Practical Guide to Interpretive Near-Infrared Spectroscopy. CRC Press Taylor and Francis Group, Boca Raton, USA (2008).