Most people don’t think about how molecules fit in the ultra-small spaces between other molecules, but that is what Professor Masahide Takahashi’s research team do every day at Osaka Metropolitan University. They study metal-organic frameworks (MOF), composed of modularly arranged metal ions and molecules (organic linkers), forming a scaffold. Metal ions act as corners connected by longer organic linkers. A MOF can be made using different metals and organic linkers, so they can be designed for specific chemical/physical properties, attractive for coating sensors in optical and electronic devices. This is because the MOF scaffold leaves a lot of internal space open. These pores can “host” numerous “guest” molecules, that can access the MOFs’ huge internal surface area, which make them ideal for developing catalytic materials, gas storage, gas separation and environmental remediation.
By using an infrared spectrometer to measure the MOF and guest molecule absorbance of two differently polarised types of infrared light, the research team’s method is the first to measure both guest-guest and guest-host interactions and do it in real-time. The additions to standard infrared spectrometers required for use with light polarisation use minimal materials, including easily replicable 3D-printed components. This represents a huge advance in MOF study, making it vastly more accessible compared to the previously used X-ray diffraction or solid-state nuclear magnetic resonance spectroscopy.
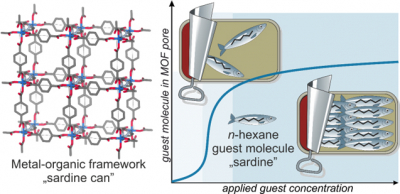
A unique property of MOFs is that they can change their conductivity and photoluminescence by increasing or decreasing the number of guest molecules that are hosted in their pores. When tightly packed in, the guest molecules can align, creating direction-dependent differences to light absorption and electrical resistance. The researchers coined this phenomenon the “sardine can” effect because the molecules in gases are not always round, differently shaped gas molecules often act like “sardines” when confined in a nanopore “can”. When long molecules are added, they bump into each other until they are side-by-side, efficiently packed and pointing in the same direction just like the sardines.
If you would shine a light through the side of a clear sardine can, you could get a good idea about the direction the sardines were aligned based their shadows. However, the MOF films and guest molecules are too small to cast shadows, so the researchers used a different feature of light: polarisation. The researchers used infrared light in two polarisations and measured the absorbance of the guest molecule for each polarisation separately. As the partial pressure of the gas in the MOF film was increased, the guest molecules began to align, increasing the absorbance of one polarisation.
This allowed the researchers to find the partial pressure where the host molecules aligned and how they interacted at different pressures. The molecular bonds between different atoms absorb specific wavelengths of infrared light. By comparing which of the polarised wavelengths were absorbed, the researchers could determine the direction molecules in the MOF film were pointing. At higher pressures, when the MOF pores were full, they also discovered defects that began to appear in the MOF scaffold due to the presence of the guest molecules. When the guest molecules were removed, the defects reversed, giving the first clear observation of interactions between guest and host molecules in the MOF.
These results are only the beginning, as this technique can be used to study different MOF films and guest molecule interactions in real-time. This new frontier of materials science has the potential to solve a lot of humanity’s future challenges. “These results clarify how molecules enter nanopores and how they are aligned. Based on this technique, we can expect to develop high-performance porous materials!”, concluded Dr Bettina Baumgartner.
















